Application of a hemolysis assay for analysis of complement activation by perfluorocarbon nanoparticles
- PMID: 24211337
- PMCID: PMC3966962
- DOI: 10.1016/j.nano.2013.10.012
Application of a hemolysis assay for analysis of complement activation by perfluorocarbon nanoparticles
Abstract
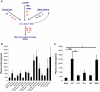
Nanoparticles offer new options for medical diagnosis and therapeutics with their capacity to specifically target cells and tissues with imaging agents and/or drug payloads. The unique physical aspects of nanoparticles present new challenges for this promising technology. Studies indicate that nanoparticles often elicit moderate to severe complement activation. Using human in vitro assays that corroborated the mouse in vivo results we previously presented mechanistic studies that define the pathway and key components involved in modulating complement interactions with several gadolinium-functionalized perfluorocarbon nanoparticles (PFOB). Here we employ a modified in vitro hemolysis-based assay developed in conjunction with the mouse in vivo model to broaden our analysis to include PFOBs of varying size, charge and surface chemistry and examine the variations in nanoparticle-mediated complement activity between individuals. This approach may provide the tools for an in-depth structure-activity relationship study that will guide the eventual development of biocompatible nanoparticles.
From the clinical editor: Unique physical aspects of nanoparticles may lead to moderate to severe complement activation in vivo, which represents a challenge to clinical applicability. In order to guide the eventual development of biocompatible nanoparticles, this team of authors report a modified in vitro hemolysis-based assay developed in conjunction with their previously presented mouse model to enable in-depth structure-activity relationship studies.
Keywords: Complement; Hemolysis Assay; Mouse Model; Nanomedicine; Nanoparticles; Perfluorocarbon.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



References
-
- Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615. - PubMed
-
- Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469. - PubMed
-
- Chanan-Khan A, et al. Complement activation following first exposure to pegylated liposomal doxorubicin (Doxil): possible role in hypersensitivity reactions. Ann Oncol. 2003;14:1430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R42 HL112518/HL/NHLBI NIH HHS/United States
- CA136398/CA/NCI NIH HHS/United States
- U54 CA136398/CA/NCI NIH HHS/United States
- R01 HL113392/HL/NHLBI NIH HHS/United States
- R01 CA154737/CA/NCI NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- R01 AR056468/AR/NIAMS NIH HHS/United States
- U01 NS073457/NS/NINDS NIH HHS/United States
- HL113392/HL/NHLBI NIH HHS/United States
- R01AI051436/AI/NIAID NIH HHS/United States
- P30 EY002687/EY/NEI NIH HHS/United States
- R01 AI051436/AI/NIAID NIH HHS/United States
- AR056468/AR/NIAMS NIH HHS/United States
- CA100623/CA/NCI NIH HHS/United States
- CA154737/CA/NCI NIH HHS/United States
- U01NS073457/NS/NINDS NIH HHS/United States
- R21 CA100623/CA/NCI NIH HHS/United States
- HL112518/HL/NHLBI NIH HHS/United States
- P30 EY 02687/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

