Performance of common analysis methods for detecting low-frequency single nucleotide variants in targeted next-generation sequence data
- PMID: 24211364
- PMCID: PMC3873500
- DOI: 10.1016/j.jmoldx.2013.09.003
Performance of common analysis methods for detecting low-frequency single nucleotide variants in targeted next-generation sequence data
Abstract
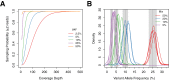
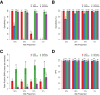
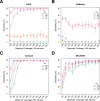
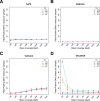
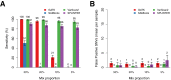
Next-generation sequencing (NGS) is becoming a common approach for clinical testing of oncology specimens for mutations in cancer genes. Unlike inherited variants, cancer mutations may occur at low frequencies because of contamination from normal cells or tumor heterogeneity and can therefore be challenging to detect using common NGS analysis tools, which are often designed for constitutional genomic studies. We generated high-coverage (>1000×) NGS data from synthetic DNA mixtures with variant allele fractions (VAFs) of 25% to 2.5% to assess the performance of four variant callers, SAMtools, Genome Analysis Toolkit, VarScan2, and SPLINTER, in detecting low-frequency variants. SAMtools had the lowest sensitivity and detected only 49% of variants with VAFs of approximately 25%; whereas the Genome Analysis Toolkit, VarScan2, and SPLINTER detected at least 94% of variants with VAFs of approximately 10%. VarScan2 and SPLINTER achieved sensitivities of 97% and 89%, respectively, for variants with observed VAFs of 1% to 8%, with >98% sensitivity and >99% positive predictive value in coding regions. Coverage analysis demonstrated that >500× coverage was required for optimal performance. The specificity of SPLINTER improved with higher coverage, whereas VarScan2 yielded more false positive results at high coverage levels, although this effect was abrogated by removing low-quality reads before variant identification. Finally, we demonstrate the utility of high-sensitivity variant callers with data from 15 clinical lung cancers.
Copyright © 2014 American Society for Investigative Pathology and the Association for Molecular Pathology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Druker B.J., Talpaz M., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., Sawyers C.L. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. - PubMed
-
- Kohl T.M., Schnittger S., Ellwart J.W., Hiddemann W., Spiekermann K. KIT exon 8 mutations associated with core-binding factor (CBF)-acute myeloid leukemia (AML) cause hyperactivation of the receptor in response to stem cell factor. Blood. 2005;105:3319–3321. - PubMed
-
- Kottaridis P.D., Gale R.E., Frew M.E., Harrison G., Langabeer S.E., Belton A.A., Walker H., Wheatley K., Bowen D.T., Burnett A.K., Goldstone A.H., Linch D.C. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. - PubMed
-
- Lièvre A., Bachet J.B., Le Corre D., Boige V., Landi B., Emile J.F., Côté J.F., Tomasic G., Penna C., Ducreux M., Rougier P., Penault-Llorca F., Laurent-Puig P. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

