Characterization of a murine type IIB procollagen-specific antibody
- PMID: 24211541
- PMCID: PMC4013248
- DOI: 10.1016/j.matbio.2013.10.014
Characterization of a murine type IIB procollagen-specific antibody
Abstract
Type II collagen is the major collagenous component of the cartilage extracellular matrix; formation of a covalently cross-linked type II collagen network provides cartilage with important tensile properties. The Col2a1 gene is encoded by 54 exons, of which exon 2 is subject to alternative splicing, resulting in different isoforms named IIA, IIB, IIC and IID. The two major procollagen protein isoforms are type IIA and type IIB procollagen. Type IIA procollagen mRNA contains exon 2 and is generated predominantly by chondroprogenitor cells and other non-cartilaginous tissues. Differentiated chondrocytes generate type IIB procollagen, devoid of exon 2. Although type IIA procollagen is produced in certain non-collagenous tissues during development, this developmentally-regulated alternative splicing switch to type IIB procollagen is restricted to cartilage cells. Though a much studied and characterized molecule, the importance of the various type II collagen protein isoforms in cartilage development and homeostasis is still not completely understood. Effective antibodies against specific epitopes of these isoforms can be useful tools to decipher function. However, most type II collagen antibodies to date recognize either all isoforms or the IIA procollagen isoform. To specifically identify the murine type IIB procollagen, we have generated a rabbit antibody (termed IIBN) directed to a peptide sequence that spans the murine exon 1-3 peptide junction. Characterization of the affinity-purified antibody by western blotting of collagens extracted from wild type murine cartilage or cartilage from Col2a1(+ex2) knock-in mice (which generates predominantly the type IIA procollagen isoform) demonstrated that the IIBN antibody is specific to the type IIB procollagen isoform. IIBN antibody was also able to detect the native type IIB procollagen in the hypertrophic chondrocytes of the wild type growth plate, but not in those of the Col2a1(+ex2) homozygous knock-in mice, by both immunofluorescence and immunohistochemical studies. Thus the IIBN antibody will permit an in-depth characterization of the distribution of IIB procollagen isoform in mouse skeletal tissues. In addition, this antibody will be an important reagent for characterizing mutant type II collagen phenotypes and for monitoring type II procollagen processing and trafficking.
Keywords: Antibody; Cartilage; Chondrocyte; Type II collagen; Type IIA procollagen; Type IIB procollagen.
Copyright © 2013 International Society of Matrix Biology. Published by Elsevier B.V. All rights reserved.
Figures

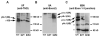



Similar articles
-
Alternative splicing of type II procollagen: IIB or not IIB?Connect Tissue Res. 2014 Jun;55(3):165-76. doi: 10.3109/03008207.2014.908860. Epub 2014 Apr 18. Connect Tissue Res. 2014. PMID: 24669942 Free PMC article. Review.
-
Changes in type II procollagen isoform expression during chondrogenesis by disruption of an alternative 5' splice site within Col2a1 exon 2.Matrix Biol. 2014 Jun;36:51-63. doi: 10.1016/j.matbio.2014.04.004. Epub 2014 Apr 13. Matrix Biol. 2014. PMID: 24735995 Free PMC article.
-
Disruption of the developmentally-regulated Col2a1 pre-mRNA alternative splicing switch in a transgenic knock-in mouse model.Matrix Biol. 2012 Apr;31(3):214-26. doi: 10.1016/j.matbio.2011.12.004. Epub 2012 Jan 9. Matrix Biol. 2012. PMID: 22248926 Free PMC article.
-
Molecular properties and fibril ultrastructure of types II and XI collagens in cartilage of mice expressing exclusively the α1(IIA) collagen isoform.Matrix Biol. 2014 Feb;34:105-13. doi: 10.1016/j.matbio.2013.09.006. Epub 2013 Oct 7. Matrix Biol. 2014. PMID: 24113490 Free PMC article.
-
Modulation of collagen synthesis in normal and osteoarthritic cartilage.Biorheology. 2004;41(3-4):535-42. Biorheology. 2004. PMID: 15299284 Review.
Cited by
-
Site-1 protease regulates skeletal stem cell population and osteogenic differentiation in mice.Biol Open. 2018 Feb 22;7(2):bio032094. doi: 10.1242/bio.032094. Biol Open. 2018. PMID: 29437042 Free PMC article.
-
Presence of type IIB procollagen in mouse articular cartilage and growth plate is revealed by immuno-histochemical analysis with a novel specific antibody.Matrix Biol Plus. 2023 Mar 5;18:100130. doi: 10.1016/j.mbplus.2023.100130. eCollection 2023 Jun. Matrix Biol Plus. 2023. PMID: 36941890 Free PMC article.
-
Alternative splicing of type II procollagen: IIB or not IIB?Connect Tissue Res. 2014 Jun;55(3):165-76. doi: 10.3109/03008207.2014.908860. Epub 2014 Apr 18. Connect Tissue Res. 2014. PMID: 24669942 Free PMC article. Review.
-
Cartilage-specific ablation of site-1 protease in mice results in the endoplasmic reticulum entrapment of type IIb procollagen and down-regulation of cholesterol and lipid homeostasis.PLoS One. 2014 Aug 22;9(8):e105674. doi: 10.1371/journal.pone.0105674. eCollection 2014. PLoS One. 2014. PMID: 25147951 Free PMC article.
References
-
- Aubert-Foucher E, Mayer N, Pasdeloup M, Pagnon A, Hartmann D, Mallein-Gerin F. A unique tool to selectively detect the chondrogenic IIB form of human type II procollagen protein. Matrix Biol. 2013 - PubMed
-
- Eyre DR, Wu JJ, Fernandes RJ, Pietka TA, Weis MA. Recent developments in cartilage research: matrix biology of the collagen II/IX/XI heterofibril network. Biochem Soc Trans. 2002;30:893–899. - PubMed
-
- Fukui N, McAlinden A, Zhu Y, Crouch E, Broekelmann TJ, Mecham RP, Sandell LJ. Processing of Type II Procollagen Amino Propeptide by Matrix Metalloproteinases. J. Biol. Chem. 2002;277:2193–2201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

