Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis
- PMID: 24212107
- PMCID: PMC3898413
- DOI: 10.1136/bmj.f6625
Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis
Abstract
Objective: To compare the efficacy and safety of biodegradable polymer drug eluting stents with those of bare metal stents and durable polymer drug eluting stents.
Design: Mixed treatment comparison meta-analysis of 258,544 patient years of follow-up from randomized trials.
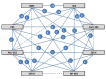
Data sources and study selection: PubMed, Embase, and Central were searched for randomized trials comparing any of the Food and Drug Administration approved durable polymer drug eluting stents (sirolimus eluting, paclitaxel eluting, cobalt chromium everolimus eluting, platinum chromium everolimus eluting, zotarolimus eluting-Endeavor, and zotarolimus eluting-Resolute) or biodegradable polymer drug eluting stents, with each other or against bare metal stents.
Outcomes: Long term efficacy (target vessel revascularization, target lesion revascularization) and safety (death, myocardial infarction, stent thrombosis). Landmark analysis at more than one year was evaluated to assess the potential late benefit of biodegradable polymer drug eluting stents.
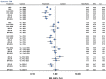
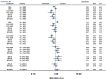
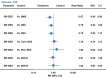
Results: From 126 randomized trials and 258,544 patient years of follow-up, for long term efficacy (target vessel revascularization), biodegradable polymer drug eluting stents were superior to paclitaxel eluting stents (rate ratio 0.66, 95% credibility interval 0.57 to 0.78) and zotarolimus eluting stent-Endeavor (0.69, 0.56 to 0.84) but not to newer generation durable polymer drug eluting stents (for example: 1.03, 0.89 to 1.21 versus cobalt chromium everolimus eluting stents). Similarly, biodegradable polymer drug eluting stents were superior to paclitaxel eluting stents (rate ratio 0.61, 0.37 to 0.89) but inferior to cobalt chromium everolimus eluting stents (2.04, 1.27 to 3.35) for long term safety (definite stent thrombosis). In the landmark analysis after one year, biodegradable polymer drug eluting stents were superior to sirolimus eluting stents for definite stent thrombosis (rate ratio 0.29, 0.10 to 0.82) but were associated with increased mortality compared with cobalt chromium everolimus eluting stents (1.52, 1.02 to 2.22). Overall, among all stent types, the newer generation durable polymer drug eluting stents (zotarolimus eluting stent-Resolute, cobalt chromium everolimus eluting stents, and platinum chromium everolimus eluting stents) were the most efficacious (lowest target vessel revascularization rate) stents, and cobalt chromium everolimus eluting stents were the safest with significant reductions in definite stent thrombosis (rate ratio 0.35, 0.21 to 0.53), myocardial infarction (0.65, 0.55 to 0.75), and death (0.72, 0.58 to 0.90) compared with bare metal stents.
Conclusions: Biodegradable polymer drug eluting stents are superior to first generation durable polymer drug eluting stents but not to newer generation durable polymer stents in reducing target vessel revascularization. Newer generation durable polymer stents, and especially cobalt chromium everolimus eluting stents, have the best combination of efficacy and safety. The utility of biodegradable polymer stents in the context of excellent clinical outcomes with newer generation durable polymer stents needs to be proven.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures



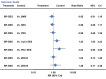

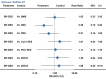
Comment in
-
Interventional cardiology: biodegradable-polymer DES versus second-generation durable-polymer DES.Nat Rev Cardiol. 2014 Jan;11(1):6. doi: 10.1038/nrcardio.2013.184. Epub 2013 Nov 26. Nat Rev Cardiol. 2014. PMID: 24275687 No abstract available.
-
Bioabsorbable drug eluting stent: winner or sinner?BMJ. 2013 Nov 27;347:f7091. doi: 10.1136/bmj.f7091. BMJ. 2013. PMID: 24284343 No abstract available.
References
-
- Bangalore S, Kumar S, Fusaro M, Amoroso N, Attubato MJ, Feit F, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation 2012;125:2873-91. - PubMed
-
- Raber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation 2012;125:1110-21. - PubMed
-
- De Luca G, Dirksen MT, Spaulding C, Kelbaek H, Schalij M, Thuesen L, et al. Drug-eluting vs bare-metal stents in primary angioplasty: a pooled patient-level meta-analysis of randomized trials. Arch Intern Med 2012;172:611-21; discussion 21-2. - PubMed
-
- Lagerqvist B, James SK, Stenestrand U, Lindbäck J, Nilsson T, Wallentin L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med 2007;356:1009-19. - PubMed
-
- Sabate M, Cequier A, Iniguez A, Serra A, Hernandez-Antolin R, Mainar V, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet 2012;380:1482-90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
