Analyses of interactions between heparin and the apical surface proteins of Plasmodium falciparum
- PMID: 24212193
- PMCID: PMC3822384
- DOI: 10.1038/srep03178
Analyses of interactions between heparin and the apical surface proteins of Plasmodium falciparum
Erratum in
- Sci Rep. 2014;4:4349
Abstract
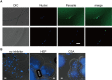
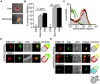
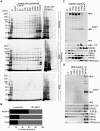
Heparin, a sulfated glycoconjugate, reportedly inhibits the blood-stage growth of the malaria parasite Plasmodium falciparum. Elucidation of the inhibitory mechanism is valuable for developing novel invasion-blocking treatments based on heparin. Merozoite surface protein 1 has been reported as a candidate target of heparin; however, to better understand the molecular mechanisms involved, we characterized the molecules that bind to heparin during merozoite invasion. Here, we show that heparin binds only at the apical tip of the merozoite surface and that multiple heparin-binding proteins localize preferentially in the apical organelles. To identify heparin-binding proteins, parasite proteins were fractionated by means of heparin affinity chromatography and subjected to immunoblot analysis with ligand-specific antibodies. All tested members of the Duffy and reticulocyte binding-like families bound to heparin with diverse affinities. These findings suggest that heparin masks the apical surface of merozoites and blocks interaction with the erythrocyte membrane after initial attachment.
Figures




References
-
- World Health Organization. World malaria report: 2012 <www.who.int/malaria> (2012).
-
- Dvorak J. A., Miller L. H., Whitehouse W. C. & Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science 187, 748–750 (1975). - PubMed
-
- Holder A. A. The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria. Parasitology 136, 1445–1456 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

