Atomically precise edge chlorination of nanographenes and its application in graphene nanoribbons
- PMID: 24212200
- PMCID: PMC3831289
- DOI: 10.1038/ncomms3646
Atomically precise edge chlorination of nanographenes and its application in graphene nanoribbons
Abstract
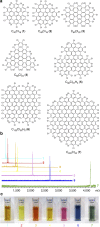
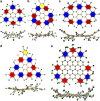
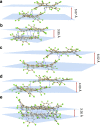
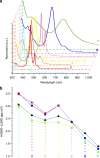
Chemical functionalization is one of the most powerful and widely used strategies to control the properties of nanomaterials, particularly in the field of graphene. However, the ill-defined structure of the present functionalized graphene inhibits atomically precise structural characterization and structure-correlated property modulation. Here we present a general edge chlorination protocol for atomically precise functionalization of nanographenes at different scales from 1.2 to 3.4 nm and its application in graphene nanoribbons. The well-defined edge chlorination is unambiguously confirmed by X-ray single-crystal analysis, which also discloses the characteristic non-planar molecular shape and detailed bond lengths of chlorinated nanographenes. Chlorinated nanographenes and graphene nanoribbons manifest enhanced solution processability associated with decreases in the optical band gap and frontier molecular orbital energy levels, exemplifying the structure-correlated property modulation by precise edge chlorination.
Figures
References
-
- Novoselov K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004). - PubMed
-
- Geim A. K. Graphene: status and prospects. Science 324, 1530–1534 (2009). - PubMed
-
- Castro Neto A. H., Guinea F., Peres N. M. R., Novoselov K. S. & Geim A. K. The electronic properties of graphene. Rev. Mod. Phys. 81, 109–162 (2009).
-
- Ritter K. A. & Lyding J. W. The influence of edge structure on the electronic properties of graphene quantum dots and nanoribbons. Nat. Mater. 8, 235–242 (2009). - PubMed
-
- Fujii S. & Enoki T. Nanographene and graphene edges: electronic structure and nanofabrication. Acc. Chem. Res. 46, 2202–2210 (2012). - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

