Polysulfides as intermediates in the oxidation of sulfide to sulfate by Beggiatoa spp
- PMID: 24212585
- PMCID: PMC3911116
- DOI: 10.1128/AEM.02852-13
Polysulfides as intermediates in the oxidation of sulfide to sulfate by Beggiatoa spp
Abstract
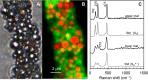
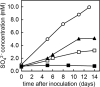
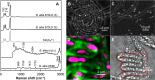
Zero-valent sulfur is a key intermediate in the microbial oxidation of sulfide to sulfate. Many sulfide-oxidizing bacteria produce and store large amounts of sulfur intra- or extracellularly. It is still not understood how the stored sulfur is metabolized, as the most stable form of S(0) under standard biological conditions, orthorhombic α-sulfur, is most likely inaccessible to bacterial enzymes. Here we analyzed the speciation of sulfur in single cells of living sulfide-oxidizing bacteria via Raman spectroscopy. Our results showed that under various ecological and physiological conditions, all three investigated Beggiatoa strains stored sulfur as a combination of cyclooctasulfur (S8) and inorganic polysulfides (Sn(2-)). Linear sulfur chains were detected during both the oxidation and reduction of stored sulfur, suggesting that Sn(2-) species represent a universal pool of bioavailable sulfur. Formation of polysulfides due to the cleavage of sulfur rings could occur biologically by thiol-containing enzymes or chemically by the strong nucleophile HS(-) as Beggiatoa migrates vertically between oxic and sulfidic zones in the environment. Most Beggiatoa spp. thus far studied can oxidize sulfur further to sulfate. Our results suggest that the ratio of produced sulfur and sulfate varies depending on the sulfide flux. Almost all of the sulfide was oxidized directly to sulfate under low-sulfide-flux conditions, whereas only 50% was oxidized to sulfate under high-sulfide-flux conditions leading to S(0) deposition. With Raman spectroscopy we could show that sulfate accumulated in Beggiatoa filaments, reaching intracellular concentrations of 0.72 to 1.73 M.
Figures



References
-
- Winogradsky S. 1887. Über Schwefelbakterien. Bot. Zeitung 45:489–610
-
- Dahl C, Prange A. 2006. Inclusions in prokaryotes, p 21–51 Springer, Berlin, Germany
-
- Brune DC. 2004. Anoxygenic photosynthetic bacteria, p 847–870 Springer, Houten, Netherlands
-
- Steudel R, Holdt G, Göbel T, Hazeu W. 1987. Chromatographic separation of higher polythionates SnO62− (n = 3…22) and their detection in cultures of Thiobacillus ferroxidans; molecular composition of bacterial sulfur secretions. Angew. Chem. Int. Ed. Engl. 26:151–153. 10.1002/anie.198701511 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

