Protein kinase a in cancer
- PMID: 24212646
- PMCID: PMC3756396
- DOI: 10.3390/cancers3010913
Protein kinase a in cancer
Abstract
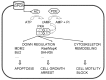
In the past, many chromosomal and genetic alterations have been examined as possible causes of cancer. However, some tumors do not display a clear molecular and/or genetic signature. Therefore, other cellular processes may be involved in carcinogenesis. Genetic alterations of proteins involved in signal transduction have been extensively studied, for example oncogenes, while modifications in intracellular compartmentalization of these molecules, or changes in the expression of unmodified genes have received less attention. Yet, epigenetic modulation of second messenger systems can deeply modify cellular functioning and in the end may cause instability of many processes, including cell mitosis. It is important to understand the functional meaning of modifications in second messenger intracellular pathways and unravel the role of downstream proteins in the initiation and growth of tumors. Within this framework, the cAMP system has been examined. cAMP is a second messenger involved in regulation of a variety of cellular functions. It acts mainly through its binding to cAMP-activated protein kinases (PKA), that were suggested to participate in the onset and progression of various tumors. PKA may represent a biomarker for tumor detection, identification and staging, and may be a potential target for pharmacological treatment of tumors.
Figures
References
-
- Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat. Rev. Cancer. 2005;5:172–183. - PubMed
-
- Rail T.W., Sutherland E.W. Formation of a cyclic adenine ribonucleotide by tissue particles. J. Biol. Chem. 1958;232:1065–1076. - PubMed
-
- Chin K.V., Yang W.L., Ravatn R., Kita T., Reitman E., Vettori D., Cvijic M.E., Shin M., Iacono L. Reinventing the wheel of cyclic AMP: Novel mechanisms of cAMP signaling. Ann. NY Acad. Sci. 2002;968:49–64. - PubMed
-
- David M., Petricoin E., III, Larner A.C. Activation of protein kinase A inhibits interferon induction of the Jak/Stat pathway in U266 cells. J. Biol. Chem. 1996;271:4585–4588. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

