Cancer genome sequencing and its implications for personalized cancer vaccines
- PMID: 24213133
- PMCID: PMC3763418
- DOI: 10.3390/cancers3044191
Cancer genome sequencing and its implications for personalized cancer vaccines
Abstract
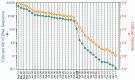
New DNA sequencing platforms have revolutionized human genome sequencing. The dramatic advances in genome sequencing technologies predict that the $1,000 genome will become a reality within the next few years. Applied to cancer, the availability of cancer genome sequences permits real-time decision-making with the potential to affect diagnosis, prognosis, and treatment, and has opened the door towards personalized medicine. A promising strategy is the identification of mutated tumor antigens, and the design of personalized cancer vaccines. Supporting this notion are preliminary analyses of the epitope landscape in breast cancer suggesting that individual tumors express significant numbers of novel antigens to the immune system that can be specifically targeted through cancer vaccines.
Figures


References
-
- Lander E.S., Linton L.M., Birren B., Nusbaum C., Zody M.C., Baldwin J., Devon K., Dewar K., Doyle M., FitzHugh W., et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. - PubMed
-
- Venter J.C., Adams M.D., Myers E.W., Li P.W., Mural R.J., Sutton G.G., Smith H.O., Yandell M., Evans C.A., Holt R.A., et al. The sequence of the human genome. Science. 2001;291:1304–1351. - PubMed
-
- Mardis E.R. A decade's perspective on DNA sequencing technology. Nature. 2011;470:198–203. - PubMed
-
- Pettersson E., Lundeberg J., Ahmadian A. Generations of sequencing technologies. Genomics. 2009;93:105–111. - PubMed
-
- Mardis E.R. Next-generation DNA sequencing methods. Annu. Rev. Genomics Hum. Genet. 2008;9:387–402. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

