Deletion of airway cilia results in noninflammatory bronchiectasis and hyperreactive airways
- PMID: 24213915
- PMCID: PMC3920206
- DOI: 10.1152/ajplung.00095.2013
Deletion of airway cilia results in noninflammatory bronchiectasis and hyperreactive airways
Abstract
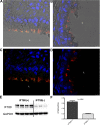
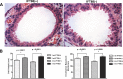
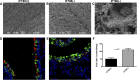
The mechanisms for the development of bronchiectasis and airway hyperreactivity have not been fully elucidated. Although genetic, acquired diseases and environmental influences may play a role, it is also possible that motile cilia can influence this disease process. We hypothesized that deletion of a key intraflagellar transport molecule, IFT88, in mature mice causes loss of cilia, resulting in airway remodeling. Airway cilia were deleted by knockout of IFT88, and airway remodeling and pulmonary function were evaluated. In IFT88(-) mice there was a substantial loss of airway cilia on respiratory epithelium. Three months after the deletion of cilia, there was clear evidence for bronchial remodeling that was not associated with inflammation or apparent defects in mucus clearance. There was evidence for airway epithelial cell hypertrophy and hyperplasia. IFT88(-) mice exhibited increased airway reactivity to a methacholine challenge and decreased ciliary beat frequency in the few remaining cells that possessed cilia. With deletion of respiratory cilia there was a marked increase in the number of club cells as seen by scanning electron microscopy. We suggest that airway remodeling may be exacerbated by the presence of club cells, since these cells are involved in airway repair. Club cells may be prevented from differentiating into respiratory epithelial cells because of a lack of IFT88 protein that is necessary to form a single nonmotile cilium. This monocilium is a prerequisite for these progenitor cells to transition into respiratory epithelial cells. In conclusion, motile cilia may play an important role in controlling airway structure and function.
Keywords: bronchiectasis; hyperreactivity; lung; respiratory.
Figures





References
-
- Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, Bell PD, Schwiebert EM, Yoder BK. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 132: 5329–5339, 2005 - PubMed
-
- Bell PD, Fitzgibbon W, Sas K, Stenbit AE, Amria M, Houston A, Reichert R, Gilley S, Siegal GP, Bissler J, Bilgen M, Chou PC, Guay-Woodford L, Yoder B, Haycraft CJ, Siroky B. Loss of primary cilia upregulates renal hypertrophic signaling and promotes cystogenesis. J Am Soc Nephrol 22: 839–848, 2011 - PMC - PubMed
-
- Cohen M, Sahn SA. Bronchiectasis in systemic diseases. Chest 116: 1063–1074, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

