The Thellungiella salsuginea tonoplast aquaporin TsTIP1;2 functions in protection against multiple abiotic stresses
- PMID: 24214268
- PMCID: PMC3894706
- DOI: 10.1093/pcp/pct166
The Thellungiella salsuginea tonoplast aquaporin TsTIP1;2 functions in protection against multiple abiotic stresses
Abstract
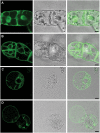
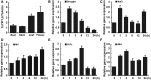
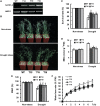
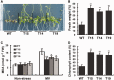
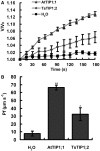
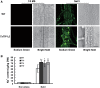
Examination of aquaporin (AQP) membrane channels in extremophile plants may increase our understanding of plant tolerance to high salt, drought or other conditions. Here, we cloned a tonoplast AQP gene (TsTIP1;2) from the halophyte Thellungiella salsuginea and characterized its biological functions. TsTIP1;2 transcripts accumulate to high levels in several organs, increasing in response to multiple external stimuli. Ectopic overexpression of TsTIP1;2 in Arabidopsis significantly increased plant tolerance to drought, salt and oxidative stresses. TsTIP1;2 had water channel activity when expressed in Xenopus oocytes. TsTIP1;2 was also able to conduct H₂O₂ molecules into yeast cells in response to oxidative stress. TsTIP1;2 was not permeable to Na(+) in Xenopus oocytes, but it could facilitate the entry of Na(+) ions into plant cell vacuoles by an indirect process under high-salinity conditions. Collectively, these data showed that TsTIP1;2 could mediate the conduction of both H₂O and H₂O₂ across membranes, and may act as a multifunctional contributor to survival of T. salsuginea in highly stressful habitats.
Keywords: Aquaporin; Channeling activity; Stress tolerance; Thellungiella salsuginea.
Figures


References
-
- Alexandersson E, Fraysse L, Sjovall-Larsen S, Gustavsson S, Fellert M, Karlsson M, et al. Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol. 2005;59:469–484. - PubMed
-
- Andrews J, Adams SR, Burton KS, Evered CE. Subcellular localization of peroxidase in tomato fruit skin and the possible implications for the regulation of fruit growth. J. Exp. Bot. 2002;53:2185–2191. - PubMed
-
- Aroca R, Porcel R, Ruiz-Lozano JM. Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot. 2012;63:43–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

