Leaf oil body functions as a subcellular factory for the production of a phytoalexin in Arabidopsis
- PMID: 24214535
- PMCID: PMC3875792
- DOI: 10.1104/pp.113.230185
Leaf oil body functions as a subcellular factory for the production of a phytoalexin in Arabidopsis
Abstract
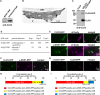
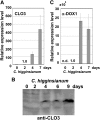
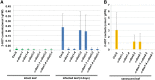
Oil bodies are intracellular structures present in the seed and leaf cells of many land plants. Seed oil bodies are known to function as storage compartments for lipids. However, the physiological function of leaf oil bodies is unknown. Here, we show that leaf oil bodies function as subcellular factories for the production of a stable phytoalexin in response to fungal infection and senescence. Proteomic analysis of oil bodies prepared from Arabidopsis (Arabidopsis thaliana) leaves identified caleosin (CLO3) and α-dioxygenase (α-DOX1). Both CLO3 and α-DOX1 were localized on the surface of oil bodies. Infection with the pathogenic fungus Colletotrichum higginsianum promoted the formation of CLO3- and α-DOX1-positive oil bodies in perilesional areas surrounding the site of infection. α-DOX1 catalyzes the reaction from α-linolenic acid (a major fatty acid component of oil bodies) to an unstable compound, 2-hydroperoxy-octadecatrienoic acid (2-HPOT). Intriguingly, a combination of α-DOX1 and CLO3 produced a stable compound, 2-hydroxy-octadecatrienoic acid (2-HOT), from α-linolenic acid. This suggests that the colocalization of α-DOX1 and CLO3 on oil bodies might prevent the degradation of unstable 2-HPOT by efficiently converting 2-HPOT into the stable compound 2-HOT. We found that 2-HOT had antifungal activity against members of the genus Colletotrichum and that infection with C. higginsianum induced 2-HOT production. These results defined 2-HOT as an Arabidopsis phytoalexin. This study provides, to our knowledge, the first evidence that leaf oil bodies produce a phytoalexin under a pathological condition, which suggests a new mechanism of plant defense.
Figures




Similar articles
-
Oil body-mediated defense against fungi: From tissues to ecology.Plant Signal Behav. 2015;10(2):e989036. doi: 10.4161/15592324.2014.989036. Plant Signal Behav. 2015. PMID: 25764319 Free PMC article.
-
Leaf oil bodies are subcellular factories producing antifungal oxylipins.Curr Opin Plant Biol. 2015 Jun;25:145-50. doi: 10.1016/j.pbi.2015.05.019. Epub 2015 Jun 5. Curr Opin Plant Biol. 2015. PMID: 26051035 Review.
-
The stress induced caleosin, RD20/CLO3, acts as a negative regulator of GPA1 in Arabidopsis.Plant Mol Biol. 2021 Oct;107(3):159-175. doi: 10.1007/s11103-021-01189-x. Epub 2021 Oct 2. Plant Mol Biol. 2021. PMID: 34599731
-
Role of 9-lipoxygenase and α-dioxygenase oxylipin pathways as modulators of local and systemic defense.Mol Plant. 2012 Jul;5(4):914-28. doi: 10.1093/mp/ssr105. Epub 2011 Dec 22. Mol Plant. 2012. PMID: 22199234
-
[The "chemical defense" of plants against pathogenic microbes: Phytoalexins biosynthesis and molecular regulations].Ying Yong Sheng Tai Xue Bao. 2020 Jul;31(7):2161-2167. doi: 10.13287/j.1001-9332.202007.018. Ying Yong Sheng Tai Xue Bao. 2020. PMID: 32715677 Review. Chinese.
Cited by
-
Regulation of Sugar and Storage Oil Metabolism by Phytochrome during De-etiolation.Plant Physiol. 2020 Feb;182(2):1114-1129. doi: 10.1104/pp.19.00535. Epub 2019 Nov 20. Plant Physiol. 2020. PMID: 31748417 Free PMC article.
-
Involvement of the caleosin/peroxygenase RD20 in the control of cell death during Arabidopsis responses to pathogens.Plant Signal Behav. 2015;10(4):e991574. doi: 10.4161/15592324.2014.991574. Plant Signal Behav. 2015. PMID: 25830533 Free PMC article.
-
Sucrose Production Mediated by Lipid Metabolism Suppresses the Physical Interaction of Peroxisomes and Oil Bodies during Germination of Arabidopsis thaliana.J Biol Chem. 2016 Sep 16;291(38):19734-45. doi: 10.1074/jbc.M116.748814. Epub 2016 Jul 27. J Biol Chem. 2016. PMID: 27466365 Free PMC article.
-
Effects of impaired steryl ester biosynthesis on tomato growth and developmental processes.Front Plant Sci. 2022 Sep 29;13:984100. doi: 10.3389/fpls.2022.984100. eCollection 2022. Front Plant Sci. 2022. PMID: 36247562 Free PMC article.
-
Evidence for early intracellular accumulation of volatile compounds during spadix development in Arum italicum L. and preliminary data on some tropical Aroids.Naturwissenschaften. 2014 Aug;101(8):623-35. doi: 10.1007/s00114-014-1197-8. Epub 2014 Jun 13. Naturwissenschaften. 2014. PMID: 24925357
References
-
- Ahuja I, Kissen R, Bones AM. (2012) Phytoalexins in defense against pathogens. Trends Plant Sci 17: 73–90 - PubMed
-
- Aubert Y, Vile D, Pervent M, Aldon D, Ranty B, Simonneau T, Vavasseur A, Galaud JP. (2010) RD20, a stress-inducible caleosin, participates in stomatal control, transpiration and drought tolerance in Arabidopsis thaliana. Plant Cell Physiol 51: 1975–1987 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

