Using mutagenesis and structural biology to map the binding site for the Plasmodium falciparum merozoite protein PfRh4 on the human immune adherence receptor
- PMID: 24214979
- PMCID: PMC3879568
- DOI: 10.1074/jbc.M113.520346
Using mutagenesis and structural biology to map the binding site for the Plasmodium falciparum merozoite protein PfRh4 on the human immune adherence receptor
Abstract
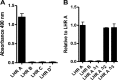
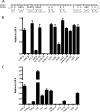
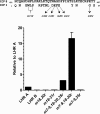
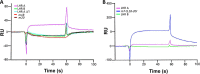
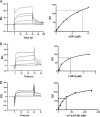
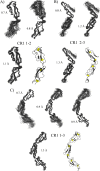
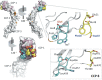
To survive and replicate within the human host, malaria parasites must invade erythrocytes. Invasion can be mediated by the P. falciparum reticulocyte-binding homologue protein 4 (PfRh4) on the merozoite surface interacting with complement receptor type 1 (CR1, CD35) on the erythrocyte membrane. The PfRh4 attachment site lies within the three N-terminal complement control protein modules (CCPs 1-3) of CR1, which intriguingly also accommodate binding and regulatory sites for the key complement activation-specific proteolytic products, C3b and C4b. One of these regulatory activities is decay-accelerating activity. Although PfRh4 does not impact C3b/C4b binding, it does inhibit this convertase disassociating capability. Here, we have employed ELISA, co-immunoprecipitation, and surface plasmon resonance to demonstrate that CCP 1 contains all the critical residues for PfRh4 interaction. We fine mapped by homologous substitution mutagenesis the PfRh4-binding site on CCP 1 and visualized it with a solution structure of CCPs 1-3 derived by NMR and small angle x-ray scattering. We cross-validated these results by creating an artificial PfRh4-binding site through substitution of putative PfRh4-interacting residues from CCP 1 into their homologous positions within CCP 8; strikingly, this engineered binding site had an ∼30-fold higher affinity for PfRh4 than the native one in CCP 1. These experiments define a candidate site on CR1 by which P. falciparum merozoites gain access to human erythrocytes in a non-sialic acid-dependent pathway of merozoite invasion.
Keywords: Cell Surface Receptor; Complement System; Convertases; Malaria; NMR; Plasmodium; Protein Structure; Reticulocyte-binding Homologue Proteins; Surface Plasmon Resonance (SPR).
Figures




References
-
- (2010) World malaria report 2010. in WHO Global Malaria Programme, World Health Organization
-
- Agnandji S. T., Lell B., Fernandes J. F., Abossolo B. P., Methogo B. G., Kabwende A. L., Adegnika A. A., Mordmuller B., Issifou S., Kremsner P. G., Sacarlal J., Aide P., Lanaspa M., Aponte J. J., Machevo S., Acacio S., Bulo H., Sigauque B., Macete E., Alonso P., Abdulla S., Salim N., Minja R., Mpina M., Ahmed S., Ali A. M., Mtoro A. T., Hamad A. S., Mutani P., Tanner M., Tinto H., D'Alessandro U., Sorgho H., Valea I., Bihoun B., Guiraud I., Kaboré B., Sombié O., Guiguemdé R. T., Ouédraogo J. B., Hamel M. J., Kariuki S., Oneko M., Odero C., Otieno K., Awino N., McMorrow M., Muturi-Kioi V., Laserson K. F., Slutsker L., Otieno W., Otieno L., Otsyula N., Gondi S., Otieno A., Owira V., Oguk E., Odongo G., Woods J. B., Ogutu B., Njuguna P., Chilengi R., Akoo P., Kerubo C., Maingi C., Lang T., Olotu A., Bejon P., Marsh K., Mwambingu G., Owusu-Agyei S., Asante K. P., Osei-Kwakye K., Boahen O., Dosoo D., Asante I., Adjei G., Kwara E., Chandramohan D., Greenwood B., Lusingu J., Gesase S., Malabeja A., Abdul O., Mahende C., Liheluka E., Malle L., Lemnge M., Theander T. G., Drakeley C., Ansong D., Agbenyega T., Adjei S., Boateng H. O., Rettig T., Bawa J., Sylverken J., Sambian D., Sarfo A., Agyekum A., Martinson F., Hoffman I., Mvalo T., Kamthunzi P., Nkomo R., Tembo T., Tegha G., Tsidya M., Kilembe J., Chawinga C., Ballou W. R., Cohen J., Guerra Y., Jongert E., Lapierre D., Leach A., Lievens M., Ofori-Anyinam O., Olivier A., Vekemans J., Carter T., Kaslow D., Leboulleux D., Loucq C., Radford A., Savarese B., Schellenberg D., Sillman M., Vansadia P. (2012) A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367, 2284–2295 - PMC - PubMed
-
- Schofield L., Grau G. E. (2005) Immunological processes in malaria pathogenesis. Nat. Rev. Immunol. 5, 722–735 - PubMed
-
- Cowman A. F., Crabb B. S. (2006) Invasion of red blood cells by malaria parasites. Cell 124, 755–766 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

