A phosphoinositide 3-kinase (PI3K)-serum- and glucocorticoid-inducible kinase 1 (SGK1) pathway promotes Kv7.1 channel surface expression by inhibiting Nedd4-2 protein
- PMID: 24214981
- PMCID: PMC3873543
- DOI: 10.1074/jbc.M113.525931
A phosphoinositide 3-kinase (PI3K)-serum- and glucocorticoid-inducible kinase 1 (SGK1) pathway promotes Kv7.1 channel surface expression by inhibiting Nedd4-2 protein
Abstract
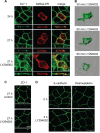
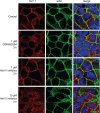
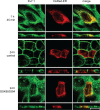
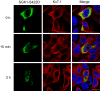
Epithelial cell polarization involves several kinase signaling cascades that eventually divide the surface membrane into an apical and a basolateral part. One kinase, which is activated during the polarization process, is phosphoinositide 3-kinase (PI3K). In MDCK cells, the basolateral potassium channel Kv7.1 requires PI3K activity for surface-expression during the polarization process. Here, we demonstrate that Kv7.1 surface expression requires tonic PI3K activity as PI3K inhibition triggers endocytosis of these channels in polarized MDCK. Pharmacological inhibition of SGK1 gave similar results as PI3K inhibition, whereas overexpression of constitutively active SGK1 overruled it, suggesting that SGK1 is the primary downstream target of PI3K in this process. Furthermore, knockdown of the ubiquitin ligase Nedd4-2 overruled PI3K inhibition, whereas a Nedd4-2 interaction-deficient Kv7.1 mutant was resistant to both PI3K and SGK1 inhibition. Altogether, these data suggest that a PI3K-SGK1 pathway stabilizes Kv7.1 surface expression by inhibiting Nedd4-2-dependent endocytosis and thereby demonstrates that Nedd4-2 is a key regulator of Kv7.1 localization and turnover in epithelial cells.
Keywords: E3 Ubiquitin Ligase; Endocytosis; Ion Channels; KCNQ1; PI 3-Kinase (PI3K); Trafficking.
Figures






References
-
- Barhanin J., Lesage F., Guillemare E., Fink M., Lazdunski M., Romey G. (1996) K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384, 78–80 - PubMed
-
- Sanguinetti M. C., Curran M. E., Zou A., Shen J., Spector P. S., Atkinson D. L., Keating M. T. (1996) Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 384, 80–83 - PubMed
-
- Ullrich S., Su J., Ranta F., Wittekindt O. H., Ris F., Rösler M., Gerlach U., Heitzmann D., Warth R., Lang F. (2005) Effects of I(Ks) channel inhibitors in insulin-secreting INS-1 cells. Pflugers. Arch. 451, 428–436 - PubMed
-
- Yamagata K., Senokuchi T., Lu M., Takemoto M., Fazlul Karim M., Go C., Sato Y., Hatta M., Yoshizawa T., Araki E., Miyazaki J., Song W. J. (2011) Voltage-gated K+ channel KCNQ1 regulates insulin secretion in MIN6 β-cell line. Biochem. Biophys. Res. Commun. 407, 620–625 - PubMed
-
- Dedek K., Waldegger S. (2001) Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers. Arch. 442, 896–902 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

