SagE induces highly effective protective immunity against Streptococcus iniae mainly through an immunogenic domain in the extracellular region
- PMID: 24215645
- PMCID: PMC3829104
- DOI: 10.1186/1751-0147-55-78
SagE induces highly effective protective immunity against Streptococcus iniae mainly through an immunogenic domain in the extracellular region
Abstract
Background: Streptococcus iniae is a Gram-positive bacterium and a severe pathogen of a wide range of farmed fish. S. iniae possesses a virulence-associated streptolysin S cluster composed of several components, one of which is SagE. SagE a transmembrane protein with one major extracellular region named ECR. This study aimed to develop a SagE-based DNA candidate vaccine against streptococcosis and examine the immunoprotective mechanism of the vaccine.
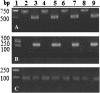
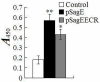
Results: We constructed a DNA vaccine, pSagE, based on the sagE gene and examined its immunological property in a Japanese flounder (Paralichthys olivaceus) model. The results showed that at 7 days post-vaccination, expression of SagE at transcription and translation levels was detected in the tissues of the vaccinated fish. After challenge with S. iniae at one and two months post-vaccination, pSagE-vaccinated fish exhibited relative percent survival (RPS) of 95% and 88% respectively. Immunological analysis showed that (i) pSagE significantly upregulated the expression of a wide range of immune genes, (ii) pSagE induced the production of specific serum antibodies that bound whole-cell S. iniae, and (iii) treatment of S. iniae with pSagE-induced antibodies blocked bacterial invasion of host cells. To localize the immunoprotective domain of SagE, the ECR-expressing DNA vaccine pSagEECR was constructed. Immunization analysis showed that flounder vaccinated with pSagEECR exhibited a RPS of 68%, and that pSagEECR induced serum antibody production and immune gene expression in a manner similar to, though to lower magnitudes than, those induced by pSagE.
Conclusions: We in this study developed a DNA vaccine, pSagE, which induces highly protective immunity against S. iniae. The protective effect of pSagE is probably due to its ability to elicit systemic immune response, in particular that of the humoral branch, which leads to production of specific serum antibodies that impair bacterial infection. These results add insights to the immunoprotective mechanism of fish DNA vaccine.
Figures

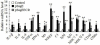

Similar articles
-
A divalent DNA vaccine based on Sia10 and OmpU induces cross protection against Streptococcus iniae and Vibrio anguillarum in Japanese flounder.Fish Shellfish Immunol. 2012 Jun;32(6):1216-22. doi: 10.1016/j.fsi.2012.03.024. Epub 2012 Mar 28. Fish Shellfish Immunol. 2012. PMID: 22480661
-
Construction and comparative study of monovalent and multivalent DNA vaccines against Streptococcus iniae.Fish Shellfish Immunol. 2012 Dec;33(6):1303-10. doi: 10.1016/j.fsi.2012.10.004. Epub 2012 Oct 11. Fish Shellfish Immunol. 2012. PMID: 23063784
-
Pellet feed adsorbed with the recombinant Lactococcus lactis BFE920 expressing SiMA antigen induced strong recall vaccine effects against Streptococcus iniae infection in olive flounder (Paralichthys olivaceus).Fish Shellfish Immunol. 2016 Aug;55:374-83. doi: 10.1016/j.fsi.2016.06.010. Epub 2016 Jun 11. Fish Shellfish Immunol. 2016. PMID: 27302864
-
Immunization with bacterial antigens: infections with streptococci and related organisms.Dev Biol Stand. 1997;90:153-60. Dev Biol Stand. 1997. PMID: 9270844 Review.
-
Towards control of Streptococcus iniae.Emerg Infect Dis. 2009 Dec;15(12):1891-6. doi: 10.3201/eid1512.090232. Emerg Infect Dis. 2009. PMID: 19961667 Free PMC article. Review.
Cited by
-
Host-Pathogen Interactions of Marine Gram-Positive Bacteria.Biology (Basel). 2022 Sep 5;11(9):1316. doi: 10.3390/biology11091316. Biology (Basel). 2022. PMID: 36138795 Free PMC article. Review.
-
Antigenic Protein Screening and Design of Multi-Epitope Vaccine Against Lactococcus garvieri and Streptococcus iniae for Combating Lactococcosis and Streptococcosis in Fish.Vet Med Sci. 2025 Jul;11(4):e70465. doi: 10.1002/vms3.70465. Vet Med Sci. 2025. PMID: 40526224 Free PMC article.
References
-
- Low DE, Liu E, Fuller J, McGeer A. Emerging Infections 3. Washington. DC: ASM Press; 1999. Streptococcus iniae: an emerging pathogen in the aquaculture industry; pp. 53–65.
-
- Austin D, Austin B. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish. Berlin: Springer; 2008. Characteristics of the diseases; p. 18.
-
- Lahav D, Eyngor M, Hurvitz A, Ghittino C, Lublin A, Eldar A. Streptococcus iniae type II infections in rainbow trout Oncorhynchus mykiss. Dis Aquat Org. 2004;62:177–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

