Induction of myelodysplasia by myeloid-derived suppressor cells
- PMID: 24216507
- PMCID: PMC3809779
- DOI: 10.1172/JCI67580
Induction of myelodysplasia by myeloid-derived suppressor cells
Abstract
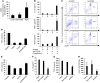
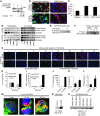
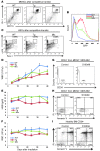
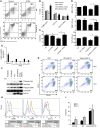
Myelodysplastic syndromes (MDS) are age-dependent stem cell malignancies that share biological features of activated adaptive immune response and ineffective hematopoiesis. Here we report that myeloid-derived suppressor cells (MDSC), which are classically linked to immunosuppression, inflammation, and cancer, were markedly expanded in the bone marrow of MDS patients and played a pathogenetic role in the development of ineffective hematopoiesis. These clonally distinct MDSC overproduce hematopoietic suppressive cytokines and function as potent apoptotic effectors targeting autologous hematopoietic progenitors. Using multiple transfected cell models, we found that MDSC expansion is driven by the interaction of the proinflammatory molecule S100A9 with CD33. These 2 proteins formed a functional ligand/receptor pair that recruited components to CD33’s immunoreceptor tyrosine-based inhibition motif (ITIM), inducing secretion of the suppressive cytokines IL-10 and TGF-β by immature myeloid cells. S100A9 transgenic mice displayed bone marrow accumulation of MDSC accompanied by development of progressive multilineage cytopenias and cytological dysplasia. Importantly, early forced maturation of MDSC by either all-trans-retinoic acid treatment or active immunoreceptor tyrosine-based activation motif–bearing (ITAM-bearing) adapter protein (DAP12) interruption of CD33 signaling rescued the hematologic phenotype. These findings indicate that primary bone marrow expansion of MDSC driven by the S100A9/CD33 pathway perturbs hematopoiesis and contributes to the development of MDS.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

