NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis
- PMID: 24216508
- PMCID: PMC3809780
- DOI: 10.1172/JCI67603
NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis
Abstract
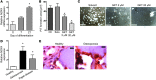
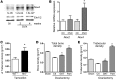
ROS are implicated in bone diseases. NADPH oxidase 4 (NOX4), a constitutively active enzymatic source of ROS, may contribute to the development of such disorders. Therefore, we studied the role of NOX4 in bone homeostasis. Nox4(-/-) mice displayed higher bone density and reduced numbers and markers of osteoclasts. Ex vivo, differentiation of monocytes into osteoclasts with RANKL and M-CSF induced Nox4 expression. Loss of NOX4 activity attenuated osteoclastogenesis, which was accompanied by impaired activation of RANKL-induced NFATc1 and c-JUN. In an in vivo model of murine ovariectomy–induced osteoporosis, pharmacological inhibition or acute genetic knockdown of Nox4 mitigated loss of trabecular bone. Human bone obtained from patients with increased osteoclast activity exhibited increased NOX4 expression. Moreover, a SNP of NOX4 was associated with elevated circulating markers of bone turnover and reduced bone density in women. Thus, NOX4 is involved in bone loss and represents a potential therapeutic target for the treatment of osteoporosis.
Figures



Comment in
-
Bone: Oxidative stress and osteoporosis.Nat Rev Endocrinol. 2014 Jan;10(1):3. doi: 10.1038/nrendo.2013.225. Epub 2013 Nov 5. Nat Rev Endocrinol. 2014. PMID: 24189509 No abstract available.
-
NADPH oxidase 4 represents a potential target for the treatment of osteoporosis.Cell Mol Immunol. 2014 Jul;11(4):317-9. doi: 10.1038/cmi.2014.9. Epub 2014 Mar 3. Cell Mol Immunol. 2014. PMID: 24583714 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

