Hirschsprung-like disease is exacerbated by reduced de novo GMP synthesis
- PMID: 24216510
- PMCID: PMC3809793
- DOI: 10.1172/JCI69781
Hirschsprung-like disease is exacerbated by reduced de novo GMP synthesis
Abstract
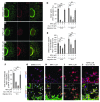
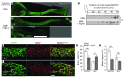
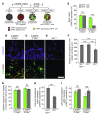
Hirschsprung disease (HSCR) is a partially penetrant oligogenic birth defect that occurs when enteric nervous system (ENS) precursors fail to colonize the distal bowel during early pregnancy. Genetic defects underlie HSCR, but much of the variability in the occurrence and severity of the birth defect remain unexplained. We hypothesized that nongenetic factors might contribute to disease development. Here we found that mycophenolate, an inhibitor of de novo guanine nucleotide biosynthesis, and 8 other drugs identified in a zebrafish screen impaired ENS development. In mice, mycophenolate treatment selectively impaired ENS precursor proliferation, delayed precursor migration, and induced bowel aganglionosis. In 2 different mouse models of HSCR, addition of mycophenolate increased the penetrance and severity of Hirschsprung-like pathology. Mycophenolate treatment also reduced ENS precursor migration as well as lamellipodia formation, proliferation, and survival in cultured enteric neural crest–derived cells. Using X-inactivation mosaicism for the purine salvage gene Hprt, we found that reduced ENS precursor proliferation most likely causes mycophenolate-induced migration defects and aganglionosis. To the best of our knowledge, mycophenolate is the first medicine identified that causes major ENS malformations and Hirschsprung-like pathology in a mammalian model. These studies demonstrate a critical role for de novo guanine nucleotide biosynthesis in ENS development and suggest that some cases of HSCR may be preventable.
Figures



References
-
- Heuckeroth RO. Pediatric Neurogastroenterology. 2013. Hirschsprung disease. In: Faure C, Di Lorenzo C, Thapar N, eds. pp. 271–283. New York, New York, USA: Humana Press;
-
- Gabriel SB, et al. Segregation at three loci explains familial and population risk in Hirschsprung disease. Nat Genet. 2002;31(1):89–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

