Chronic itch development in sensory neurons requires BRAF signaling pathways
- PMID: 24216512
- PMCID: PMC3809799
- DOI: 10.1172/JCI70528
Chronic itch development in sensory neurons requires BRAF signaling pathways
Abstract
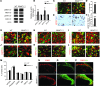
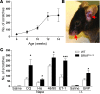
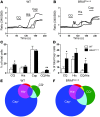
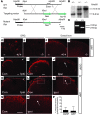
Chronic itch, or pruritus, is associated with a wide range of skin abnormalities. The mechanisms responsible for chronic itch induction and persistence remain unclear. We developed a mouse model in which a constitutively active form of the serine/threonine kinase BRAF was expressed in neurons gated by the sodium channel Nav1.8 (BRAF(Nav1.8) mice). We found that constitutive BRAF pathway activation in BRAF(Nav1.8) mice results in ectopic and enhanced expression of a cohort of itch-sensing genes, including gastrin-releasing peptide (GRP) and MAS-related GPCR member A3 (MRGPRA3), in nociceptors expressing transient receptor potential vanilloid 1 (TRPV1). BRAF(Nav1.8) mice showed de novo neuronal responsiveness to pruritogens, enhanced pruriceptor excitability, and heightened evoked and spontaneous scratching behavior. GRP receptor expression was increased in the spinal cord, indicating augmented coding capacity for itch subsequent to amplified pruriceptive inputs. Enhanced GRP expression and sustained ERK phosphorylation were observed in sensory neurons of mice with allergic contact dermatitis– or dry skin–elicited itch; however, spinal ERK activation was not required for maintaining central sensitization of itch. Inhibition of either BRAF or GRP signaling attenuated itch sensation in chronic itch mouse models. These data uncover RAF/MEK/ERK signaling as a key regulator that confers a subset of nociceptors with pruriceptive properties to initiate and maintain long-lasting itch sensation.
Figures




Similar articles
-
Sensitization of spinal itch transmission neurons in a mouse model of chronic itch requires an astrocytic factor.J Allergy Clin Immunol. 2020 Jan;145(1):183-191.e10. doi: 10.1016/j.jaci.2019.09.034. Epub 2019 Nov 28. J Allergy Clin Immunol. 2020. PMID: 31787267
-
Central opioid receptors mediate morphine-induced itch and chronic itch via disinhibition.Brain. 2021 Mar 3;144(2):665-681. doi: 10.1093/brain/awaa430. Brain. 2021. PMID: 33367648
-
A subpopulation of nociceptors specifically linked to itch.Nat Neurosci. 2013 Feb;16(2):174-82. doi: 10.1038/nn.3289. Epub 2012 Dec 23. Nat Neurosci. 2013. PMID: 23263443 Free PMC article.
-
Mas-Related G Protein-Coupled Receptors and the Biology of Itch Sensation.Annu Rev Genet. 2017 Nov 27;51:103-121. doi: 10.1146/annurev-genet-120116-024723. Annu Rev Genet. 2017. PMID: 29178819 Review.
-
New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms?Pflugers Arch. 2013 Dec;465(12):1671-85. doi: 10.1007/s00424-013-1284-2. Epub 2013 May 1. Pflugers Arch. 2013. PMID: 23636773 Free PMC article. Review.
Cited by
-
Structures of human gastrin-releasing peptide receptors bound to antagonist and agonist for cancer and itch therapy.Proc Natl Acad Sci U S A. 2023 Feb 7;120(6):e2216230120. doi: 10.1073/pnas.2216230120. Epub 2023 Feb 1. Proc Natl Acad Sci U S A. 2023. PMID: 36724251 Free PMC article.
-
Why we scratch an itch: the molecules, cells and circuits of itch.Nat Neurosci. 2014 Feb;17(2):175-82. doi: 10.1038/nn.3619. Epub 2014 Jan 28. Nat Neurosci. 2014. PMID: 24473265 Free PMC article. Review.
-
Neuroimmune interactions in itch: Do chronic itch, chronic pain, and chronic cough share similar mechanisms?Pulm Pharmacol Ther. 2015 Dec;35:81-6. doi: 10.1016/j.pupt.2015.09.001. Epub 2015 Sep 6. Pulm Pharmacol Ther. 2015. PMID: 26351759 Free PMC article. Review.
-
Primary afferent and spinal cord expression of gastrin-releasing peptide: message, protein, and antibody concerns.J Neurosci. 2015 Jan 14;35(2):648-57. doi: 10.1523/JNEUROSCI.2955-14.2015. J Neurosci. 2015. PMID: 25589759 Free PMC article.
-
Chemogenetic activation of central gastrin-releasing peptide-expressing neurons elicits itch-related scratching behavior in male and female mice.Pharmacol Res Perspect. 2021 May;9(3):e00790. doi: 10.1002/prp2.790. Pharmacol Res Perspect. 2021. PMID: 34000759 Free PMC article.
References
-
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4(1):72–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

