Label-free quantitative protein profiling of vastus lateralis muscle during human aging
- PMID: 24217021
- PMCID: PMC3879620
- DOI: 10.1074/mcp.M113.032698
Label-free quantitative protein profiling of vastus lateralis muscle during human aging
Abstract
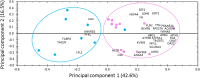
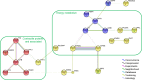
Sarcopenia corresponds to the loss of muscle mass occurring during aging, and is associated with a loss of muscle functionality. Proteomic links the muscle functional changes with protein expression pattern. To better understand the mechanisms involved in muscle aging, we performed a proteomic analysis of Vastus lateralis muscle in mature and older women. For this, a shotgun proteomic method was applied to identify soluble proteins in muscle, using a combination of high performance liquid chromatography and mass spectrometry. A label-free protein profiling was then conducted to quantify proteins and compare profiles from mature and older women. This analysis showed that 35 of the 366 identified proteins were linked to aging in muscle. Most of the proteins were under-represented in older compared with mature women. We built a functional interaction network linking the proteins differentially expressed between mature and older women. The results revealed that the main differences between mature and older women were defined by proteins involved in energy metabolism and proteins from the myofilament and cytoskeleton. This is the first time that label-free quantitative proteomics has been applied to study of aging mechanisms in human skeletal muscle. This approach highlights new elements for elucidating the alterations observed during aging and may lead to novel sarcopenia biomarkers.
Figures


References
-
- Cruz-Jentoft A. J., Baeyens J. P., Bauer J. M., Boirie Y., Cederholm T., Landi F., Martin F. C., Michel J. P., Rolland Y., Schneider S. M., Topinkova E., Vandewoude M., Zamboni M. (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423 - PMC - PubMed
-
- Szulc P., Munoz F., Marchand F., Chapurlat R., Delmas P. D. (2010) Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. American Journal of Clinical Nutrition 91(5), 1227–1236 - PubMed
-
- Piec I., Listrat A., Alliot J., Chambon C., Taylor R. G., Bechet D. (2005) Differential proteome analysis of aging in rat skeletal muscle. FASEB J. 19, 1143–1145 - PubMed
-
- Doran P., Gannon J., O'Connell K., Ohlendieck K. (2007) Aging skeletal muscle shows a drastic decrease in the small heat shock proteins αB-crystallin/HspB5 and cvHsp/HspB7. Eur. J. Cell Biol.. 86, 629–640 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

