The EORTC emotional functioning computerized adaptive test: phases I-III of a cross-cultural item bank development
- PMID: 24217943
- PMCID: PMC3974657
- DOI: 10.1002/pon.3427
The EORTC emotional functioning computerized adaptive test: phases I-III of a cross-cultural item bank development
Abstract
Background: The European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Group is currently developing computerized adaptive testing measures for the Quality of Life Questionnaire Core-30 (QLQ-C30) scales. The work presented here describes the development of an EORTC item bank for emotional functioning (EF), which is one of the core domains of the QLQ-C30.
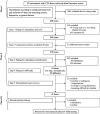
Methods: According to the EORTC guidelines on module development, the development of the EF item bank comprised four phases, of which the phases I-III are reported in the present paper. Phase I involved defining the theoretical framework for the EF item bank and a literature search. Phase II included pre-defined item selection steps and a multi-stage expert review process. In phase III, feedback from cancer patients from different countries was obtained.
Results: On the basis of literature search in phase I, a list of 1750 items was generated. These were reviewed and further developed in phase II with a focus on relevance, redundancy, clarity, and difficulty. The development and selection steps led to a preliminary list of 41 items. In phase III, patient interviews (N = 41; Austria, Denmark, Italy, and the UK) were conducted with the preliminary item list, resulting in some minor changes to item wording. The final list comprised 38 items.
Discussion: The phases I-III of the developmental process have resulted in an EF item list that was well accepted by patients in several countries. The items will be subjected to larger-scale field testing in order to establish their psychometric characteristics and their fit to an item response theory model.
Keywords: cancer; computer adaptive testing; emotional functioning; item response theory; oncology; quality of life.
© 2013 The Authors. Psycho-Oncology published by John Wiley & Sons, Ltd.
Figures
Similar articles
-
Development of an item bank for the EORTC Role Functioning Computer Adaptive Test (EORTC RF-CAT).Health Qual Life Outcomes. 2016 May 6;14:72. doi: 10.1186/s12955-016-0475-x. Health Qual Life Outcomes. 2016. PMID: 27150974 Free PMC article.
-
Cross-cultural development of an item list for computer-adaptive testing of fatigue in oncological patients.Health Qual Life Outcomes. 2011 Mar 29;9:19. doi: 10.1186/1477-7525-9-19. Health Qual Life Outcomes. 2011. PMID: 21447160 Free PMC article.
-
An emotional functioning item bank of 24 items for computerized adaptive testing (CAT) was established.J Clin Epidemiol. 2016 Feb;70:90-100. doi: 10.1016/j.jclinepi.2015.09.002. Epub 2015 Sep 9. J Clin Epidemiol. 2016. PMID: 26363341
-
Cross-cultural development of the EORTC QLQ-SWB36: a stand-alone measure of spiritual wellbeing for palliative care patients with cancer.Palliat Med. 2013 May;27(5):457-69. doi: 10.1177/0269216312451950. Epub 2012 Jul 27. Palliat Med. 2013. PMID: 22843128 Review.
-
An international update of the EORTC questionnaire for assessing quality of life in breast cancer patients: EORTC QLQ-BR45.Ann Oncol. 2020 Feb;31(2):283-288. doi: 10.1016/j.annonc.2019.10.027. Epub 2019 Dec 18. Ann Oncol. 2020. PMID: 31959345
Cited by
-
Pragmatic measurement of health satisfaction in people with type 2 diabetes mellitus using the Current Health Satisfaction Questionnaire.Patient Relat Outcome Meas. 2015 Mar 26;6:103-15. doi: 10.2147/PROM.S79368. eCollection 2015. Patient Relat Outcome Meas. 2015. PMID: 25870519 Free PMC article.
-
Prospective multicentre cohort study of patient-reported outcomes and complications following major abdominal neoplastic surgery (PATRONUS) - study protocol for a CHIR-Net student-initiated German medical audit study (CHIR-Net SIGMA study).BMC Surg. 2018 Oct 29;18(1):90. doi: 10.1186/s12893-018-0422-3. BMC Surg. 2018. PMID: 30373596 Free PMC article.
-
Impact of the COVID-19 Pandemic on Patient-Reported Outcomes of Breast Cancer Patients and Survivors.JNCI Cancer Spectr. 2020 Nov 5;5(1):pkaa104. doi: 10.1093/jncics/pkaa104. eCollection 2021 Feb. JNCI Cancer Spectr. 2020. PMID: 33437925 Free PMC article.
-
Using the emotional functioning in clinical practice to detect psychological distress in patients with advanced thoracic and colorectal cancer.Health Qual Life Outcomes. 2023 Feb 17;21(1):15. doi: 10.1186/s12955-023-02099-w. Health Qual Life Outcomes. 2023. PMID: 36800957 Free PMC article.
-
The Diabetes Intention, Attitude, and Behavior Questionnaire: evaluation of a brief questionnaire to measure physical activity, dietary control, maintenance of a healthy weight, and psychological antecedents.Patient Prefer Adherence. 2016 Feb 29;10:213-22. doi: 10.2147/PPA.S94878. eCollection 2016. Patient Prefer Adherence. 2016. PMID: 27013867 Free PMC article.
References
-
- Turk D, Dworkinb RH, Allen RR. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–345. - PubMed
-
- Zebrack B, Burg MA, Vaitones V. Distress screening: an opportunity for enhancing quality cancer care and promoting the oncology social work profession. J Psychosoc Oncol. 2012;30(6):615–624. - PubMed
-
- Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5(1):99–103. - PubMed
-
- Smith AB, Wright EP, Rush R, et al. Rasch analysis of the dimensional structure of the Hospital Anxiety and Depression Scale. Psycho-Oncology. 2006;15(9):817–827. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical