Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells
- PMID: 24218138
- PMCID: PMC3832925
- DOI: 10.1084/jem.20130323
Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells
Abstract
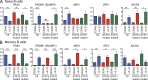
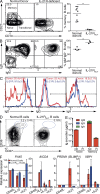
Long-lived antibody memory is mediated by the combined effects of long-lived plasma cells (PCs) and memory B cells generated in response to T cell-dependent antigens (Ags). IL-10 and IL-21 can activate multiple signaling pathways, including STAT1, STAT3, and STAT5; ERK; PI3K/Akt, and potently promote human B cell differentiation. We previously showed that loss-of-function mutations in STAT3, but not STAT1, abrogate IL-10- and IL-21-mediated differentiation of human naive B cells into plasmablasts. We report here that, in contrast to naive B cells, STAT3-deficient memory B cells responded to these STAT3-activating cytokines, differentiating into plasmablasts and secreting high levels of IgM, IgG, and IgA, as well as Ag-specific IgG. This was associated with the induction of the molecular machinery necessary for PC formation. Mutations in IL21R, however, abolished IL-21-induced responses of both naive and memory human B cells and compromised memory B cell formation in vivo. These findings reveal a key role for IL-21R/STAT3 signaling in regulating human B cell function. Furthermore, our results indicate that the threshold of STAT3 activation required for differentiation is lower in memory compared with naive B cells, thereby identifying an intrinsic difference in the mechanism underlying differentiation of naive versus memory B cells.
Figures





References
-
- Asao H., Okuyama C., Kumaki S., Ishii N., Tsuchiya S., Foster D., Sugamura K. 2001. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 167:1–5 - PubMed
-
- Avery D.T., Ellyard J.I., Mackay F., Corcoran L.M., Hodgkin P.D., Tangye S.G. 2005. Increased expression of CD27 on activated human memory B cells correlates with their commitment to the plasma cell lineage. J. Immunol. 174:4034–4042 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

