RAFT-based tri-component fluorescent glycopolymers: synthesis, characterization and application in lectin-mediated bacterial binding study
- PMID: 24218180
- PMCID: PMC3901943
- DOI: 10.1007/s10719-013-9508-4
RAFT-based tri-component fluorescent glycopolymers: synthesis, characterization and application in lectin-mediated bacterial binding study
Abstract
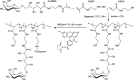
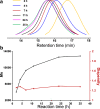
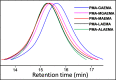
A group of fluorescent statistical glycopolymers, prepared via reversible addition-fragmentation chain-transfer (RAFT)-based polymerizations, were successfully employed in lectin-mediated bacterial binding studies. The resultant glycopolymers contained three different monomers: N-(2-hydroxyethyl) acrylamide (HEAA), N-(2-aminoethyl) methacrylamide (AEMA) and N-(2-glyconamidoethyl)-methacrylamides possessing different pendant sugars. Low dispersities (≤1.32) and predictable degrees of polymerization were observed among the products. After the polymerization, the glycopolymers were further modified by different succinimidyl ester fluorophores targeting the primary amine groups on AEMA. With their binding specificities being confirmed by testing with lectin coated agarose beads, the glycopolymers were employed in bacterial binding studies, where polymers containing α-galactose or β-galactose as the pendant sugar were specifically bound by two clinically important pathogens Pseudomonas aeruginosa and Staphylococcus aureus, respectively. This is the first report of using RAFT-based glycopolymers in bacterial binding studies, and the ready access to tri-component statistical glycopolymers also warrants further exploration of their utility in other glycobiological applications.
Figures





References
-
- Wolfenden ML, Cloninger MJ. Multivalency in carbohydrate binding. In: Wang B, Boons G-J, editors. Carbohydrate Recognition: Biological Problems, Methods, and Applications. Hoboken: Wiley; 2011. pp. 349–370.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

