Long-term impact of interferon beta-1b in patients with CIS: 8-year follow-up of BENEFIT
- PMID: 24218527
- PMCID: PMC4215285
- DOI: 10.1136/jnnp-2013-306222
Long-term impact of interferon beta-1b in patients with CIS: 8-year follow-up of BENEFIT
Abstract
Objective: To examine the long-term impact of early treatment initiation of interferon beta-1b (IFNB1b, Betaferon/Betaseron) in patients with a first event suggestive of multiple sclerosis (MS).
Methods: In the original placebo-controlled phase of BENEFIT, patients were randomised to IFNB1b 250 μg or placebo subcutaneously every other day. After 2 years or diagnosis of clinically definite MS (CDMS), all patients were offered open-label IFNB1b treatment for a maximum duration of 5 years. Thereafter, patients were enrolled in an observational extension study for up to 8.7 years.
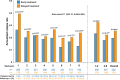
Results: Of the initial 468 patients, 284 (60.7%; IFNB1b: 178 (61.0% of the original arm), placebo: 106 (60.2% of original arm)) were enrolled in the extension study. 94.2% of patients were receiving IFNB1b. Patients originally randomised to IFNB1b had a reduced risk of developing CDMS by 32.2% over the 8-year observation period (HR 0.678; 95% CI 0.525 to 0.875; p=0.0030), a longer median time to CDMS by 1345 days (95% CI 389 to 2301), and a lower annualised relapse rate (0.196 (95% CI 0.176 to 0.218) versus 0.255 (95% CI 0.226 to 0.287), p=0.0012), with differences mainly emerging in the first year of the study. Cognitive outcomes remained higher in the early treated patients. EDSS remained low over time with a median of 1.5 in both arms.
Conclusions: These 8-year results provide further evidence supporting early initiation of treatment with IFNB1b in patients with a first event suggestive of MS.
Keywords: INTERFERON; INTERVENTIONAL; MULTIPLE SCLEROSIS; RANDOMISED TRIALS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


Comment in
-
BENEFIT 8-year results provide further support for the long-term value of early treatment of multiple sclerosis.J Neurol Neurosurg Psychiatry. 2014 Nov;85(11):1179. doi: 10.1136/jnnp-2013-306720. Epub 2014 Apr 4. J Neurol Neurosurg Psychiatry. 2014. PMID: 24706944 No abstract available.
References
-
- Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med 2000;343:938–52. - PubMed
-
- Ebers GC, Reder AT, Traboulsee A, et al. Long-term follow-up of the original interferon-beta1b trial in multiple sclerosis: design and lessons from a 16-year observational study. Clin Ther 2009;31:1724–36. - PubMed
-
- Kappos L, Freedman MS, Polman CH, et al. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 2007;370:389–97. - PubMed
-
- Kappos L, Freedman MS, Polman CH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 2009;8:987–97. - PubMed
-
- Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006;67:1242–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
