Revised standards for statistical evidence
- PMID: 24218581
- PMCID: PMC3845140
- DOI: 10.1073/pnas.1313476110
Revised standards for statistical evidence
Abstract
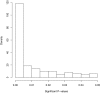
Recent advances in Bayesian hypothesis testing have led to the development of uniformly most powerful Bayesian tests, which represent an objective, default class of Bayesian hypothesis tests that have the same rejection regions as classical significance tests. Based on the correspondence between these two classes of tests, it is possible to equate the size of classical hypothesis tests with evidence thresholds in Bayesian tests, and to equate P values with Bayes factors. An examination of these connections suggest that recent concerns over the lack of reproducibility of scientific studies can be attributed largely to the conduct of significance tests at unjustifiably high levels of significance. To correct this problem, evidence thresholds required for the declaration of a significant finding should be increased to 25-50:1, and to 100-200:1 for the declaration of a highly significant finding. In terms of classical hypothesis tests, these evidence standards mandate the conduct of tests at the 0.005 or 0.001 level of significance.
Conflict of interest statement
The author declares no conflict of interest.
Figures
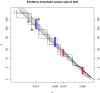
 , and binomial tests) or approximately the same (t tests) rejection regions. The smooth curves represent, from Top to Bottom, t tests based on 20, 30, and 60 degrees of freedom, the z test, and the χ2 test on 1 degree of freedom. The discontinuous curves reflect the correspondence between tests of a binomial proportion based on 20, 30, or 60 observations when the null hypothesis is p0 = 0.5.
, and binomial tests) or approximately the same (t tests) rejection regions. The smooth curves represent, from Top to Bottom, t tests based on 20, 30, and 60 degrees of freedom, the z test, and the χ2 test on 1 degree of freedom. The discontinuous curves reflect the correspondence between tests of a binomial proportion based on 20, 30, or 60 observations when the null hypothesis is p0 = 0.5.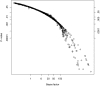
Comment in
-
Reproducibility issues in science, is P value really the only answer?Proc Natl Acad Sci U S A. 2014 May 13;111(19):E1934. doi: 10.1073/pnas.1323051111. Epub 2014 Apr 23. Proc Natl Acad Sci U S A. 2014. PMID: 24760820 Free PMC article. No abstract available.
-
Revised evidence for statistical standards.Proc Natl Acad Sci U S A. 2014 May 13;111(19):E1933. doi: 10.1073/pnas.1322995111. Epub 2014 Apr 23. Proc Natl Acad Sci U S A. 2014. PMID: 24760821 Free PMC article. No abstract available.
-
Adaptive revised standards for statistical evidence.Proc Natl Acad Sci U S A. 2014 May 13;111(19):E1935. doi: 10.1073/pnas.1322191111. Epub 2014 Apr 23. Proc Natl Acad Sci U S A. 2014. PMID: 24760822 Free PMC article. No abstract available.
-
Reply to Gelman, Gaudart, Pericchi: More reasons to revise standards for statistical evidence.Proc Natl Acad Sci U S A. 2014 May 13;111(19):E1936-7. doi: 10.1073/pnas.1400338111. Proc Natl Acad Sci U S A. 2014. PMID: 24940581 Free PMC article. No abstract available.
References
-
- Zimmer C. (April 16, 2012) A sharp rise in retractions prompts calls for reform. NY Times, Science Section.
-
- Naik G. (December 2, 2011) Scientists’ elusive goal: Reproducing study results. Wall Street Journal, Health Section.
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. - PubMed
-
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. - PubMed
-
- Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294(2):218–228. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

