Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase-encoding genes confers strong resistance to Fusarium species
- PMID: 24218613
- PMCID: PMC3845197
- DOI: 10.1073/pnas.1306373110
Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase-encoding genes confers strong resistance to Fusarium species
Abstract
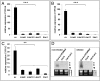
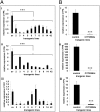
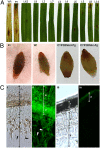
Head blight, which is caused by mycotoxin-producing fungi of the genus Fusarium, is an economically important crop disease. We assessed the potential of host-induced gene silencing targeting the fungal cytochrome P450 lanosterol C-14α-demethylase (CYP51) genes, which are essential for ergosterol biosynthesis, to restrict fungal infection. In axenic cultures of Fusarium graminearum, in vitro feeding of CYP3RNA, a 791-nt double-stranded (ds)RNA complementary to CYP51A, CYP51B, and CYP51C, resulted in growth inhibition [half-maximum growth inhibition (IC50) = 1.2 nM] as well as altered fungal morphology, similar to that observed after treatment with the azole fungicide tebuconazole, for which the CYP51 enzyme is a target. Expression of the same dsRNA in Arabidopsis and barley rendered susceptible plants highly resistant to fungal infection. Microscopic analysis revealed that mycelium formation on CYP3RNA-expressing leaves was restricted to the inoculation sites, and that inoculated barley caryopses were virtually free of fungal hyphae. This inhibition of fungal growth correlated with in planta production of siRNAs corresponding to the targeted CYP51 sequences, as well as highly efficient silencing of the fungal CYP51 genes. The high efficiency of fungal inhibition suggests that host-induced gene-silencing targeting of the CYP51 genes is an alternative to chemical treatments for the control of devastating fungal diseases.
Keywords: Fusarium head blight; HIGS; RNA interference; crop protection; small interfering RNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Osborne LE, Stein JM. Epidemiology of Fusarium head blight on small-grain cereals. Int J Food Microbiol. 2007;119(1-2):103–108. - PubMed
-
- Zhang JB, et al. Determination of the trichothecene mycotoxin chemotypes and associated geographical distribution and phylogenetic species of the Fusarium graminearum clade from China. Mycol Res. 2007;111(Pt 8):967–975. - PubMed
-
- Merhej J, Richard-Forget F, Barreau C. Regulation of trichothecene biosynthesis in Fusarium: Recent advances and new insights. Appl Microbiol Biotechnol. 2011;91(3):519–528. - PubMed
-
- Döll S, Dänicke S. The Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) in animal feeding. Prev Vet Med. 2011;102(2):132–145. - PubMed
-
- Rocha O, Ansari K, Doohan FM. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit Contam. 2005;22(4):369–378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

