Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo
- PMID: 24218625
- PMCID: PMC3845114
- DOI: 10.1073/pnas.1310065110
Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo
Abstract
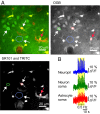
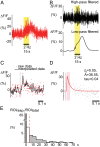
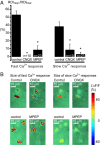
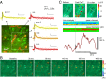
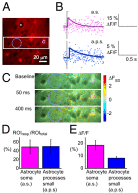
Increased neuron and astrocyte activity triggers increased brain blood flow, but controversy exists over whether stimulation-induced changes in astrocyte activity are rapid and widespread enough to contribute to brain blood flow control. Here, we provide evidence for stimulus-evoked Ca(2+) elevations with rapid onset and short duration in a large proportion of cortical astrocytes in the adult mouse somatosensory cortex. Our improved detection of the fast Ca(2+) signals is due to a signal-enhancing analysis of the Ca(2+) activity. The rapid stimulation-evoked Ca(2+) increases identified in astrocyte somas, processes, and end-feet preceded local vasodilatation. Fast Ca(2+) responses in both neurons and astrocytes correlated with synaptic activity, but only the astrocytic responses correlated with the hemodynamic shifts. These data establish that a large proportion of cortical astrocytes have brief Ca(2+) responses with a rapid onset in vivo, fast enough to initiate hemodynamic responses or influence synaptic activity.
Keywords: Ca2+ imaging; functional imaging; neurovascular coupling; sensory barrel cortex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10(11):1369–1376. - PubMed
-
- Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32(3):160–169. - PubMed
-
- Lauritzen M. Reading vascular changes in brain imaging: Is dendritic calcium the key? Nat Rev Neurosci. 2005;6(1):77–85. - PubMed
-
- Nedergaard M, Rodríguez JJ, Verkhratsky A. Glial calcium and diseases of the nervous system. Cell Calcium. 2010;47(2):140–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

