Patterns in blood pressure medication use in US incident dialysis patients over the first 6 months
- PMID: 24219348
- PMCID: PMC3840675
- DOI: 10.1186/1471-2369-14-249
Patterns in blood pressure medication use in US incident dialysis patients over the first 6 months
Abstract
Background: Several observational studies have evaluated the effect of a single exposure window with blood pressure (BP) medications on outcomes in incident dialysis patients, but whether BP medication prescription patterns remain stable or a single exposure window design is adequate to evaluate effect on outcomes is unclear.
Methods: We described patterns of BP medication prescription over 6 months after dialysis initiation in hemodialysis and peritoneal dialysis patients, stratified by cardiovascular comorbidity, diabetes, and other patient characteristics. The cohort included 13,072 adult patients (12,159 hemodialysis, 913 peritoneal dialysis) who initiated dialysis in Dialysis Clinic, Inc., facilities January 1, 2003-June 30, 2008, and remained on the original modality for at least 6 months. We evaluated monthly patterns in BP medication prescription over 6 months and at 12 and 24 months after initiation.
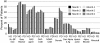
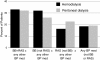
Results: Prescription patterns varied by dialysis modality over the first 6 months; substantial proportions of patients with prescriptions for beta-blockers, renin angiotensin system agents, and dihydropyridine calcium channel blockers in month 6 no longer had prescriptions for these medications by month 24. Prescription of specific medication classes varied by comorbidity, race/ethnicity, and age, but little by sex. The mean number of medications was 2.5 at month 6 in hemodialysis and peritoneal dialysis cohorts.
Conclusions: This study evaluates BP medication patterns in both hemodialysis and peritoneal dialysis patients over the first 6 months of dialysis. Our findings highlight the challenges of assessing comparative effectiveness of a single BP medication class in dialysis patients. Longitudinal designs should be used to account for changes in BP medication management over time, and designs that incorporate common combinations should be considered.
Figures

References
-
- U.S. Renal Data System. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. 2012. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

