Mutation for nonsyndromic mental retardation in the trans-2-enoyl-CoA reductase TER gene involved in fatty acid elongation impairs the enzyme activity and stability, leading to change in sphingolipid profile
- PMID: 24220030
- PMCID: PMC3868783
- DOI: 10.1074/jbc.M113.493221
Mutation for nonsyndromic mental retardation in the trans-2-enoyl-CoA reductase TER gene involved in fatty acid elongation impairs the enzyme activity and stability, leading to change in sphingolipid profile
Abstract
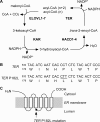
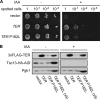
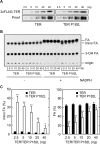
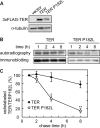
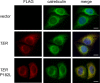
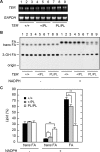
Very long-chain fatty acids (VLCFAs, chain length >C20) exist in tissues throughout the body and are synthesized by repetition of the fatty acid (FA) elongation cycle composed of four successive enzymatic reactions. In mammals, the TER gene is the only gene encoding trans-2-enoyl-CoA reductase, which catalyzes the fourth reaction in the FA elongation cycle. The TER P182L mutation is the pathogenic mutation for nonsyndromic mental retardation. This mutation substitutes a leucine for a proline residue at amino acid 182 in the TER enzyme. Currently, the mechanism by which the TER P182L mutation causes nonsyndromic mental retardation is unknown. To understand the effect of this mutation on the TER enzyme and VLCFA synthesis, we have biochemically characterized the TER P182L mutant enzyme using yeast and mammalian cells transfected with the TER P182L mutant gene and analyzed the FA elongation cycle in the B-lymphoblastoid cell line with the homozygous TER P182L mutation (TER(P182L/P182L) B-lymphoblastoid cell line). We have found that TER P182L mutant enzyme exhibits reduced trans-2-enoyl-CoA reductase activity and protein stability, thereby impairing VLCFA synthesis and, in turn, altering the sphingolipid profile (i.e. decreased level of C24 sphingomyelin and C24 ceramide) in the TER(P182L/P182L) B-lymphoblastoid cell line. We have also found that in addition to the TER enzyme-catalyzed fourth reaction, the third reaction in the FA elongation cycle is affected by the TER P182L mutation. These findings provide new insight into the biochemical defects associated with this genetic mutation.
Keywords: Fatty Acid; Fatty Acid Metabolism; Lipids; Membrane; Sphingolipid.
Figures


References
-
- Guillou H., Zadravec D., Martin P. G., Jacobsson A. (2010) The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid Res. 49, 186–199 - PubMed
-
- Kihara A. (2012) Very long-chain fatty acids: elongation, physiology and related disorders. J. Biochem. 152, 387–395 - PubMed
-
- Nugteren D. H. (1965) The enzymic chain elongation of fatty acids by rat-liver microsomes. Biochim. Biophys. Acta 106, 280–290 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

