Nitric oxide activation of guanylate cyclase pushes the α1 signaling helix and the β1 heme-binding domain closer to the substrate-binding site
- PMID: 24220034
- PMCID: PMC3879570
- DOI: 10.1074/jbc.M113.504472
Nitric oxide activation of guanylate cyclase pushes the α1 signaling helix and the β1 heme-binding domain closer to the substrate-binding site
Abstract
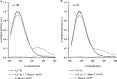
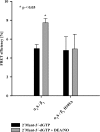
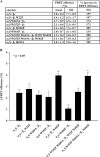
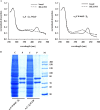
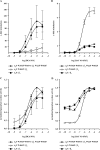
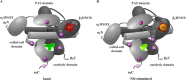
The complete structure of the assembled domains of nitric oxide-sensitive guanylate cyclase (NOsGC) remains to be determined. It is also unknown how binding of NO to heme in guanylate cyclase is communicated to the catalytic domain. In the current study the conformational change of guanylate cyclase on activation by NO was studied using FRET. Endogenous tryptophan residues were used as donors, the substrate analog 2'-Mant-3'-dGTP as acceptor. The enzyme contains five tryptophan residues distributed evenly over all four functional domains. This provides a unique opportunity to detect the movement of the functional domains relative to the substrate-binding catalytic region. FRET measurements indicate that NO brings tryptophan 22 in the αB helix of the β1 heme NO binding domain and tryptophan 466 in the second short helix of the α1 coiled-coil domain closer to the catalytic domain. We propose that the respective domains act as a pair of tongs forcing the catalytic domain into the nitric oxide-activated conformation.
Keywords: Ciguate; Cyclic GMP (cGMP); Fluorescence Resonance Energy Transfer (FRET); Guanylate Cyclase (Guanylyl Cyclase); Nitric Oxide; Signal Transduction.
Figures

References
-
- Derbyshire E. R., Marletta M. A. (2012) Structure and regulation of soluble guanylate cyclase. Annu. Rev. Biochem. 81, 533–559 - PubMed
-
- Ogawa H., Qiu Y., Ogata C. M., Misono K. S. (2004) Crystal structure of hormone-bound atrial natriuretic peptide receptor extracellular domain: rotation mechanism for transmembrane signal transduction. J. Biol. Chem. 279, 28625–28631 - PubMed
-
- Padayatti P. S., Pattanaik P., Ma X., van den Akker F. (2004) Structural insights into the regulation and the activation mechanism of mammalian guanylyl cyclases. Pharmacol. Ther. 104, 83–99 - PubMed
-
- Winger J. A., Marletta M. A. (2005) Expression and characterization of the catalytic domains of soluble guanylate cyclase: interaction with the heme domain. Biochemistry 44, 4083–4090 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

