The biophysics and cell biology of lipid droplets
- PMID: 24220094
- PMCID: PMC4526153
- DOI: 10.1038/nrm3699
The biophysics and cell biology of lipid droplets
Abstract
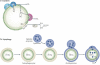
Lipid droplets are intracellular organelles that are found in most cells, where they have fundamental roles in metabolism. They function prominently in storing oil-based reserves of metabolic energy and components of membrane lipids. Lipid droplets are the dispersed phase of an oil-in-water emulsion in the aqueous cytosol of cells, and the importance of basic biophysical principles of emulsions for lipid droplet biology is now being appreciated. Because of their unique architecture, with an interface between the dispersed oil phase and the aqueous cytosol, specific mechanisms underlie their formation, growth and shrinkage. Such mechanisms enable cells to use emulsified oil when the demands for metabolic energy or membrane synthesis change. The regulation of the composition of the phospholipid surfactants at the surface of lipid droplets is crucial for lipid droplet homeostasis and protein targeting to their surfaces.
Figures
References
-
- Thiele C, Spandl J. Cell biology of lipid droplets. Curr Opin Cell Biol. 2008;20:378–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

