An NT4/TrkB-dependent increase in innervation links early-life allergen exposure to persistent airway hyperreactivity
- PMID: 24221086
- PMCID: PMC3898648
- DOI: 10.1096/fj.13-238212
An NT4/TrkB-dependent increase in innervation links early-life allergen exposure to persistent airway hyperreactivity
Abstract
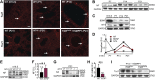
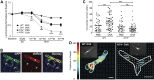
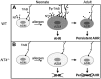
Children who are exposed to environmental respiratory insults often develop asthma that persists into adulthood. In this study, we used a neonatal mouse model of ovalbumin (OVA)-induced allergic airway inflammation to understand the long-term effects of early childhood insults on airway structure and function. We showed that OVA sensitization and challenge in early life led to a 2-fold increase in airway smooth muscle (ASM) innervation (P<0.05) and persistent airway hyperreactivity (AHR). In contrast, OVA exposure in adult life elicited short-term AHR without affecting innervation levels. We found that postnatal ASM innervation required neurotrophin (NT)-4 signaling through the TrkB receptor and that early-life OVA exposure significantly elevated NT4 levels and TrkB signaling by 5- and 2-fold, respectively, to increase innervation. Notably, blockade of NT4/TrkB signaling in OVA-exposed pups prevented both acute and persistent AHR without affecting baseline airway function or inflammation. Furthermore, biophysical assays using lung slices and isolated cells demonstrated that NT4 was necessary for hyperreactivity of ASM induced by early-life OVA exposure. Together, our findings show that the NT4/TrkB-dependent increase in innervation plays a critical role in the alteration of the ASM phenotype during postnatal growth, thereby linking early-life allergen exposure to persistent airway dysfunction.
Keywords: ASM; childhood asthma; contractility; innervation; lung slice; smooth muscle.
Figures




References
-
- Maddox L., Schwartz D. A. (2002) The pathophysiology of asthma. Annu. Rev. Med. 53, 477–498 - PubMed
-
- Martinez F. D. (2009) The connection between early life wheezing and subsequent asthma: the viral march. Allergol. Immunopathol. (Madr.) 37, 249–251 - PubMed
-
- Bisgaard H., Bønnelykke K. (2010) Long-term studies of the natural history of asthma in childhood. J. Allergy Clin. Immunol. 126, 187–197 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

