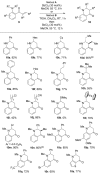
2-Aminobenzaldehydes as versatile substrates for rhodium-catalyzed alkyne hydroacylation: application to dihydroquinolone synthesis
- PMID: 24222398
- PMCID: PMC4227557
- DOI: 10.1002/anie.201308127
2-Aminobenzaldehydes as versatile substrates for rhodium-catalyzed alkyne hydroacylation: application to dihydroquinolone synthesis
Keywords: aldehydes; amines; dihydroquinolones; enones; rhodium catalysis.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

