A retrograde trafficking inhibitor of ricin and Shiga-like toxins inhibits infection of cells by human and monkey polyomaviruses
- PMID: 24222489
- PMCID: PMC3892778
- DOI: 10.1128/mBio.00729-13
A retrograde trafficking inhibitor of ricin and Shiga-like toxins inhibits infection of cells by human and monkey polyomaviruses
Abstract
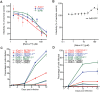
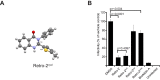
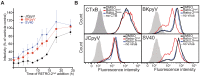
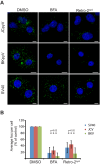
Polyomaviruses are ubiquitous pathogens that cause severe disease in immunocompromised individuals. JC polyomavirus (JCPyV) is the causative agent of the fatal demyelinating disease progressive multifocal leukoencephalopathy (PML), whereas BK polyomavirus (BKPyV) causes polyomavirus-induced nephropathy and hemorrhagic cystitis. Vaccines or antiviral therapies targeting these viruses do not exist, and treatments focus on reducing the underlying causes of immunosuppression. We demonstrate that retro-2(cycl), an inhibitor of ricin and Shiga-like toxins (SLTs), inhibits infection by JCPyV, BKPyV, and simian virus 40. Retro-2(cycl) inhibits retrograde transport of polyomaviruses to the endoplasmic reticulum, a step necessary for productive infection. Retro-2(cycl) likely inhibits polyomaviruses in a way similar to its ricin and SLT inhibition, suggesting an overlap in the cellular host factors used by bacterial toxins and polyomaviruses. This work establishes retro-2(cycl) as a potential antiviral therapy that broadly inhibits polyomaviruses and possibly other pathogens that use retrograde trafficking.
Importance: The human polyomaviruses JC polyomavirus (JCPyV) and BK polyomavirus (BKPyV) cause rare but severe diseases in individuals with reduced immune function. During immunosuppression, JCPyV disseminates from the kidney to the central nervous system and destroys oligodendrocytes, resulting in the fatal disease progressive multifocal leukoencephalopathy. Kidney transplant recipients are at increased risk of BKPyV-induced nephropathy, which results in kidney necrosis and loss of the transplanted organ. There are currently no effective therapies for JCPyV and BKPyV. We show that a small molecule named retro-2(cycl) protects cells from infection with JCPyV and BKPyV by inhibiting intracellular viral transport. Retro-2(cycl) treatment reduces viral spreading in already established infections and may therefore be able to control infection in affected patients. Further optimization of retro-2(cycl) may result in the development of an effective antiviral therapy directed toward pathogens that use retrograde trafficking to infect their hosts.
Figures
Similar articles
-
Inhibition of JC polyomavirus infectivity by the retrograde transport inhibitor Retro-2.1.Microbiol Immunol. 2020 Dec;64(12):783-791. doi: 10.1111/1348-0421.12851. Epub 2020 Dec 1. Microbiol Immunol. 2020. PMID: 32965709
-
Inhibition of Retrograde Transport Limits Polyomavirus Infection In Vivo.mSphere. 2017 Nov 15;2(6):e00494-17. doi: 10.1128/mSphereDirect.00494-17. eCollection 2017 Nov-Dec. mSphere. 2017. PMID: 29152583 Free PMC article.
-
Structural optimization of a retrograde trafficking inhibitor that protects cells from infections by human polyoma- and papillomaviruses.Bioorg Med Chem. 2014 Sep 1;22(17):4836-47. doi: 10.1016/j.bmc.2014.06.053. Epub 2014 Jul 10. Bioorg Med Chem. 2014. PMID: 25087050 Free PMC article.
-
JC polyomavirus attachment, entry, and trafficking: unlocking the keys to a fatal infection.J Neurovirol. 2015 Dec;21(6):601-13. doi: 10.1007/s13365-014-0272-4. Epub 2014 Jul 31. J Neurovirol. 2015. PMID: 25078361 Free PMC article. Review.
-
Human BK and JC polyomaviruses: Molecular insights and prevalence in Asia.Virus Res. 2020 Mar;278:197860. doi: 10.1016/j.virusres.2020.197860. Epub 2020 Jan 3. Virus Res. 2020. PMID: 31911182 Review.
Cited by
-
Taking the Scenic Route: Polyomaviruses Utilize Multiple Pathways to Reach the Same Destination.Viruses. 2020 Oct 15;12(10):1168. doi: 10.3390/v12101168. Viruses. 2020. PMID: 33076363 Free PMC article. Review.
-
Phosphoinositide 3'-Kinase γ Facilitates Polyomavirus Infection.Viruses. 2020 Oct 20;12(10):1190. doi: 10.3390/v12101190. Viruses. 2020. PMID: 33092168 Free PMC article.
-
Retro-2 protects cells from ricin toxicity by inhibiting ASNA1-mediated ER targeting and insertion of tail-anchored proteins.Elife. 2019 Nov 1;8:e48434. doi: 10.7554/eLife.48434. Elife. 2019. PMID: 31674906 Free PMC article.
-
Rab18: new insights into the function of an essential protein.Cell Mol Life Sci. 2019 May;76(10):1935-1945. doi: 10.1007/s00018-019-03050-3. Epub 2019 Mar 4. Cell Mol Life Sci. 2019. PMID: 30830238 Free PMC article. Review.
-
Susceptibility of Primary Human Choroid Plexus Epithelial Cells and Meningeal Cells to Infection by JC Virus.J Virol. 2018 Mar 28;92(8):e00105-18. doi: 10.1128/JVI.00105-18. Print 2018 Apr 15. J Virol. 2018. PMID: 29437972 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

