Evaluating the immunogenicity of protein drugs by applying in vitro MHC binding data and the immune epitope database and analysis resource
- PMID: 24222776
- PMCID: PMC3816028
- DOI: 10.1155/2013/467852
Evaluating the immunogenicity of protein drugs by applying in vitro MHC binding data and the immune epitope database and analysis resource
Abstract
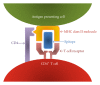
The immune system has evolved to become highly specialized in recognizing and responding to pathogens and foreign molecules. Specifically, the function of HLA class II is to ensure that a sufficient sample of peptides derived from foreign molecules is presented to T cells. This leads to an important concern in human drug development as the possible immunogenicity of biopharmaceuticals, especially those intended for chronic administration, can lead to reduced efficacy and an undesired safety profile for biological therapeutics. As part of this review, we will highlight the molecular basis of antigen presentation as a key step in the induction of T cell responses, emphasizing the events associated with peptide binding to polymorphic and polygenic HLA class II molecules. We will further review methodologies that predict HLA class II binding peptides and candidate epitopes. We will focus on tools provided by the Immune Epitope Database and Analysis Resource, discussing the basic features of different prediction methods, the objective evaluation of prediction quality, and general guidelines for practical use of these tools. Finally the use, advantages, and limitations of the methodology will be demonstrated in a review of two previous studies investigating the immunogenicity of erythropoietin and timothy grass pollen.
Figures

References
-
- Kropshofer H, Singer T. Overview of cell-based tools for pre-clinical assessment of immunogenicity of biotherapeutics. Journal of Immunotoxicology. 2006;3(3):131–136. - PubMed
-
- Wolbink GJ, Aarden LA, Dijkmans BAC. Dealing with immunogenicity of biologicals: assessment and clinical relevance. Current Opinion in Rheumatology. 2009;21(3):211–215. - PubMed
-
- Guidance for industry: Immunogenicity assessment for therapeutic protein products. Food and Drug Administration, U.S. Department of Health and Human Services, 2013.
-
- Murphy K. Janeway’s Immunobiology. 8th edition. New York, NY, USA: Garland Science; 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

