Transparency in evidence evaluation and formulary decision-making: from conceptual development to real-world implementation
- PMID: 24222979
- PMCID: PMC3814436
Transparency in evidence evaluation and formulary decision-making: from conceptual development to real-world implementation
Abstract
Objective: Establishing a better understanding of the relationship between evidence evaluation and formulary decision-making has important implications for patients, payers, and providers. The goal of our study was to develop and test a structured approach to evidence evaluation to increase clarity, consistency, and transparency in formulary decision-making.
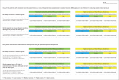
Study design: The study comprised three phases. First, an expert panel identified key constructs to formulary decision-making and created an evidence-assessment tool. Second, with the use of a balanced incomplete block design, the tool was validated by a large group of decision-makers. Third, the tool was pilot-tested in a real-world P&T committee environment.
Methods: An expert panel identified key factors associated with formulary access by rating the level of access that they would give a drug in various hypothetical scenarios. These findings were used to formulate an evidence-assessment tool that was externally validated by surveying a larger sample of decision-makers. Last, the tool was pilot-tested in a real-world environment where P&T committees used it to review new drugs.
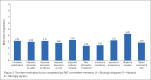
Results: Survey responses indicated that a structured approach in the formulary decision-making process could yield greater clarity, consistency, and transparency in decision-making; however, pilot-testing of the structured tool in a real-world P&T committee environment highlighted some of the limitations of our structured approach.
Conclusion: Although a structured approach to formulary decision-making is beneficial for patients, health care providers, and other stakeholders, this benefit was not realized in a real-world environment. A method to improve clarity, consistency, and transparency is still needed.
Keywords: access; decision-making; evidence; formulary.
Figures


References
-
- Department of Health and Human Services Patient Protection and Affordable Care Act. Establishment of exchanges and qualified health plans: Proposed rule. Fed Reg. 2011;76:41866–41927. Available at: www.gpo.gov/fdsys/pkg/FR-2011-07-15/html/2011-17610.htm. Accessed June 26, 2013.
-
- Eddy D. Reflections on science, judgment, and value in evidence-based decision making: A conversation with David Eddy by Sean R. Tunis. Health Aff (Millwood) 2007;26(4):w500–w515. - PubMed
-
- Eddy DM. Clinical decision making: From theory to practice. Anatomy of a decision. JAMA. 1990;263(3):441–443. - PubMed
-
- Bozzette SA, D’Amato R, Morton SC, et al. Pharmaceutical Technology Assessment for Managed Care: Current Practice and Suggestions for Improvement. Santa Monica, Calif: RAND Corp; 2001.
-
- Olsen L, Aisner D, McGinnis J. IOM Roundtable on Evidence-Based Medicine. The Learning Healthcare System: Workshop Summary. Washington, D.C: National Academies Press; 2007. - PubMed
LinkOut - more resources
Full Text Sources
