New strategies to prolong the in vivo life span of iron-based contrast agents for MRI
- PMID: 24223101
- PMCID: PMC3819506
- DOI: 10.1371/journal.pone.0078542
New strategies to prolong the in vivo life span of iron-based contrast agents for MRI
Abstract
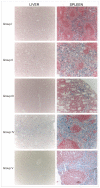
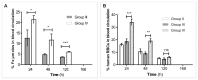
Superparamagnetic iron oxide (SPIO) and ultra small superparamagnetic iron oxide (USPIO) nanoparticles have been developed as magnetic resonance imaging (MRI) contrast agents. Iron oxide nanoparticles, that become superparamagnetic if the core particle diameter is ~ 30 nm or less, present R1 and R2 relaxivities which are much higher than those of conventional paramagnetic gadolinium chelates. Generally, these magnetic particles are coated with biocompatible polymers that prevent the agglomeration of the colloidal suspension and improve their blood distribution profile. In spite of their potential as MRI blood contrast agents, the biomedical application of iron oxide nanoparticles is still limited because of their intravascular half-life of only few hours; such nanoparticles are rapidly cleared from the bloodstream by macrophages of the reticulo-endothelial system (RES). To increase the life span of these MRI contrast agents in the bloodstream we proposed the encapsulation of SPIO nanoparticles in red blood cells (RBCs) through the transient opening of cell membrane pores. We have recently reported results obtained by applying our loading procedure to several SPIO nanoparticles with different chemical physical characteristics such as size and coating agent. In the current investigation we showed that the life span of iron-based contrast agents in the mice bloodstream was prolonged to 12 days after the intravenous injection of murine SPIO-loaded RBCs. Furthermore, we developed an animal model that implicates the pretreatment of animals with clodronate to induce a transient suppression of tissue macrophages, followed by the injection of human SPIO-loaded RBCs which make it possible to encapsulate nanoparticle concentrations (5.3-16.7 mM Fe) higher than murine SPIO-loaded RBCs (1.4-3.55 mM Fe). The data showed that, when human RBCs are used as more capable SPIO nanoparticle containers combined with a depletion of tissue macrophages, Fe concentration in animal blood is 2-3 times higher than iron concentration obtained by the use of murine SPIO-loaded RBCs.
Conflict of interest statement
Figures







References
-
- Neuberger T, Schopf B, Hofmann H, Hofmann M, Von Rechenberg B (2005) Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J Magn Magn Mater 293: 483-496. doi:10.1016/j.jmmm.2005.01.064. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

