Use of nonelectrolytes reveals the channel size and oligomeric constitution of the Borrelia burgdorferi P66 porin
- PMID: 24223145
- PMCID: PMC3819385
- DOI: 10.1371/journal.pone.0078272
Use of nonelectrolytes reveals the channel size and oligomeric constitution of the Borrelia burgdorferi P66 porin
Erratum in
- PLoS One. 2014;9(8):e105916
Abstract
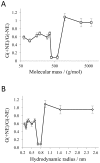
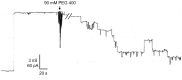
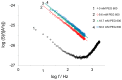
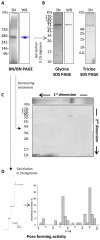
In the Lyme disease spirochete Borrelia burgdorferi, the outer membrane protein P66 is capable of pore formation with an atypical high single-channel conductance of 11 nS in 1 M KCl, which suggested that it could have a larger diameter than 'normal' Gram-negative bacterial porins. We studied the diameter of the P66 channel by analyzing its single-channel conductance in black lipid bilayers in the presence of different nonelectrolytes with known hydrodynamic radii. We calculated the filling of the channel with these nonelectrolytes and the results suggested that nonelectrolytes (NEs) with hydrodynamic radii of 0.34 nm or smaller pass through the pore, whereas neutral molecules with greater radii only partially filled the channel or were not able to enter it at all. The diameter of the entrance of the P66 channel was determined to be ≤1.9 nm and the channel has a central constriction of about 0.8 nm. The size of the channel appeared to be symmetrical as judged from one-sidedness of addition of NEs. Furthermore, the P66-induced membrane conductance could be blocked by 80-90% by the addition of the nonelectrolytes PEG 400, PEG 600 and maltohexaose to the aqueous phase in the low millimolar range. The analysis of the power density spectra of ion current through P66 after blockage with these NEs revealed no chemical reaction responsible for channel block. Interestingly, the blockage of the single-channel conductance of P66 by these NEs occurred in about eight subconductance states, indicating that the P66 channel could be an oligomer of about eight individual channels. The organization of P66 as a possible octamer was confirmed by Blue Native PAGE and immunoblot analysis, which both demonstrated that P66 forms a complex with a mass of approximately 460 kDa. Two dimension SDS PAGE revealed that P66 is the only polypeptide in the complex.
Conflict of interest statement
Figures

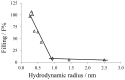
 for each nonelectrolyte was calculated according to Eq. 3. Lines are best fits to the experimental points. The channel filling of maltose, PEG 400 and PEG 600 was not included in this diagram, because the calculated values of
for each nonelectrolyte was calculated according to Eq. 3. Lines are best fits to the experimental points. The channel filling of maltose, PEG 400 and PEG 600 was not included in this diagram, because the calculated values of  and
and  were unreasonably high and could not be used due to possible interactions of these compounds with the channel interior (for details see text). The horizontal lines connect the points derived from measurements in the presence of PEG 1,000, PEG 3,000 and PEG 6,000. The other line regression was used to describe the points for the nonelectrolytes with radii ranging from 0.26 nm to 0.6 nm (ethylenglycol, glycerol, arabinose, sorbitol, PEG200, PEG300). Hydrodynamic radii of the nonelectrolytes were taken from table 2.
were unreasonably high and could not be used due to possible interactions of these compounds with the channel interior (for details see text). The horizontal lines connect the points derived from measurements in the presence of PEG 1,000, PEG 3,000 and PEG 6,000. The other line regression was used to describe the points for the nonelectrolytes with radii ranging from 0.26 nm to 0.6 nm (ethylenglycol, glycerol, arabinose, sorbitol, PEG200, PEG300). Hydrodynamic radii of the nonelectrolytes were taken from table 2.
Similar articles
-
P66 porins are present in both Lyme disease and relapsing fever spirochetes: a comparison of the biophysical properties of P66 porins from six Borrelia species.Biochim Biophys Acta. 2010 Jun;1798(6):1197-203. doi: 10.1016/j.bbamem.2010.02.011. Epub 2010 Feb 25. Biochim Biophys Acta. 2010. PMID: 20188698
-
Structural modeling and physicochemical characterization provide evidence that P66 forms a β-barrel in the Borrelia burgdorferi outer membrane.J Bacteriol. 2014 Feb;196(4):859-72. doi: 10.1128/JB.01236-13. Epub 2013 Dec 6. J Bacteriol. 2014. PMID: 24317399 Free PMC article.
-
Study of the protein complex, pore diameter, and pore-forming activity of the Borrelia burgdorferi P13 porin.J Biol Chem. 2014 Jul 4;289(27):18614-24. doi: 10.1074/jbc.M113.539528. Epub 2014 May 13. J Biol Chem. 2014. PMID: 24825899 Free PMC article.
-
The Oms66 (p66) protein is a Borrelia burgdorferi porin.Infect Immun. 1997 Sep;65(9):3654-61. doi: 10.1128/iai.65.9.3654-3661.1997. Infect Immun. 1997. PMID: 9284133 Free PMC article.
-
Pores from mitochondrial outer membranes of yeast and a porin-deficient yeast mutant: a comparison.J Bioenerg Biomembr. 1989 Aug;21(4):439-50. doi: 10.1007/BF00762516. J Bioenerg Biomembr. 1989. PMID: 2478530 Review.
Cited by
-
Pore forming activity of the potent RTX-toxin produced by pediatric pathogen Kingella kingae: Characterization and comparison to other RTX-family members.Biochim Biophys Acta. 2015 Jul;1848(7):1536-44. doi: 10.1016/j.bbamem.2015.03.036. Epub 2015 Apr 7. Biochim Biophys Acta. 2015. PMID: 25858109 Free PMC article.
-
Choreography of Lyme Disease Spirochete Adhesins To Promote Vascular Escape.Microbiol Spectr. 2023 Aug 17;11(4):e0125423. doi: 10.1128/spectrum.01254-23. Epub 2023 May 31. Microbiol Spectr. 2023. PMID: 37255427 Free PMC article.
-
Mechanisms of Viscous Media Effects on Elementary Steps of Bacterial Bioluminescent Reaction.Int J Mol Sci. 2021 Aug 17;22(16):8827. doi: 10.3390/ijms22168827. Int J Mol Sci. 2021. PMID: 34445534 Free PMC article.
-
Toxins Secreted by Bacillus Isolated from Lung Adenocarcinomas Favor the Penetration of Toxic Substances.Front Microbiol. 2015 Nov 23;6:1301. doi: 10.3389/fmicb.2015.01301. eCollection 2015. Front Microbiol. 2015. PMID: 26635767 Free PMC article.
-
Borrelia burgdorferi Keeps Moving and Carries on: A Review of Borrelial Dissemination and Invasion.Front Immunol. 2017 Feb 21;8:114. doi: 10.3389/fimmu.2017.00114. eCollection 2017. Front Immunol. 2017. PMID: 28270812 Free PMC article. Review.
References
-
- Barcena-Uribarri I, Thein M, Sacher A, Bunikis I, Bonde M, et al. (2010) P66 porins are present in both Lyme disease and relapsing fever spirochetes: a comparison of the biophysical properties of P66 porins from six Borrelia species. Biochim Biophys Acta 1798: 1197–1203. - PubMed
-
- Coburn J, Chege W, Magoun L, Bodary SC, Leong JM (1999) Characterization of a candidate Borrelia burgdorferi beta3-chain integrin ligand identified using a phage display library. Mol Microbiol 34: 926–940. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

