High-resolution structural and functional assessments of cerebral microvasculature using 3D Gas ΔR2*-mMRA
- PMID: 24223773
- PMCID: PMC3817180
- DOI: 10.1371/journal.pone.0078186
High-resolution structural and functional assessments of cerebral microvasculature using 3D Gas ΔR2*-mMRA
Abstract
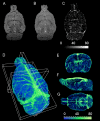
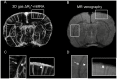
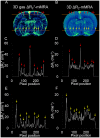
The ability to evaluate the cerebral microvascular structure and function is crucial for investigating pathological processes in brain disorders. Previous angiographic methods based on blood oxygen level-dependent (BOLD) contrast offer appropriate visualization of the cerebral vasculature, but these methods remain to be optimized in order to extract more comprehensive information. This study aimed to integrate the advantages of BOLD MRI in both structural and functional vascular assessments. The BOLD contrast was manipulated by a carbogen challenge, and signal changes in gradient-echo images were computed to generate ΔR2* maps. Simultaneously, a functional index representing the regional cerebral blood volume was derived by normalizing the ΔR2* values of a given region to those of vein-filled voxels of the sinus. This method is named 3D gas ΔR2*-mMRA (microscopic MRA). The advantages of using 3D gas ΔR2*-mMRA to observe the microvasculature include the ability to distinguish air-tissue interfaces, a high vessel-to-tissue contrast, and not being affected by damage to the blood-brain barrier. A stroke model was used to demonstrate the ability of 3D gas ΔR2*-mMRA to provide information about poststroke revascularization at 3 days after reperfusion. However, this technique has some limitations that cannot be overcome and hence should be considered when it is applied, such as magnifying vessel sizes and predominantly revealing venous vessels.
Conflict of interest statement
Figures




References
-
- Quaegebeur A, Lange C, Carmeliet P (2011) The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron 71: 406–424. - PubMed
-
- Potente M, Gerhardt H, Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146: 873–887. - PubMed
-
- Gursoy-Ozdemir Y, Yemisci M, Dalkara T (2012) Microvascular protection is essential for successful neuroprotection in stroke. J Neurochem 123 Suppl 22–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

