Mesenchymal stem cells (MSC) prevented the progression of renovascular hypertension, improved renal function and architecture
- PMID: 24223811
- PMCID: PMC3817204
- DOI: 10.1371/journal.pone.0078464
Mesenchymal stem cells (MSC) prevented the progression of renovascular hypertension, improved renal function and architecture
Abstract
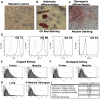
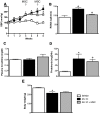
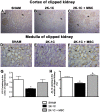
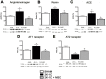
Renovascular hypertension induced by 2 Kidney-1 Clip (2K-1C) is a renin-angiotensin-system (RAS)-dependent model, leading to renal vascular rarefaction and renal failure. RAS inhibitors are not able to reduce arterial pressure (AP) and/or preserve the renal function, and thus, alternative therapies are needed. Three weeks after left renal artery occlusion, fluorescently tagged mesenchymal stem cells (MSC) (2×10(5) cells/animal) were injected weekly into the tail vein in 2K-1C hypertensive rats. Flow cytometry showed labeled MSC in the cortex and medulla of the clipped kidney. MSC prevented a further increase in the AP, significantly reduced proteinuria and decreased sympathetic hyperactivity in 2K-1C rats. Renal function parameters were unchanged, except for an increase in urinary volume observed in 2K-1C rats, which was not corrected by MSC. The treatment improved the morphology and decreased the fibrotic areas in the clipped kidney and also significantly reduced renal vascular rarefaction typical of 2K-1C model. Expression levels of IL-1β, TNF-α angiotensinogen, ACE, and Ang II receptor AT1 were elevated, whereas AT2 levels were decreased in the medulla of the clipped kidney. MSC normalized these expression levels. In conclusion, MSC therapy in the 2K-1C model (i) prevented the progressive increase of AP, (ii) improved renal morphology and microvascular rarefaction, (iii) reduced fibrosis, proteinuria and inflammatory cytokines, (iv) suppressed the intrarenal RAS, iv) decreased sympathetic hyperactivity in anesthetized animals and v) MSC were detected at the CNS suggesting that the cells crossed the blood-brain barrier. This therapy may be a promising strategy to treat renovascular hypertension and its renal consequences in the near future.
Conflict of interest statement
Figures


References
-
- Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, et al. (2002) Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 106: 1165–1171. - PubMed
-
- Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, et al. (2003) Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol 23: 1295–1301. - PubMed
-
- Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, et al.. (2006) Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. Faseb J 20: 1706 –1708. - PubMed
-
- Ploth DW (1983) Angiotensin-dependent renal mechanisms in two-kidney one-clip renal vascular hypertension. Am J Physiol 245: 131–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

