Mouse pancreas tissue slice culture facilitates long-term studies of exocrine and endocrine cell physiology in situ
- PMID: 24223842
- PMCID: PMC3817072
- DOI: 10.1371/journal.pone.0078706
Mouse pancreas tissue slice culture facilitates long-term studies of exocrine and endocrine cell physiology in situ
Abstract
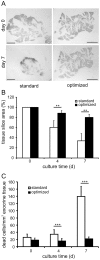
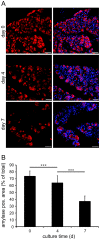
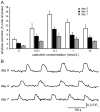
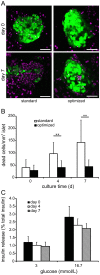
Studies on pancreatic cell physiology rely on the investigation of exocrine and endocrine cells in vitro. Particularly, in the case of the exocrine tissue these studies have suffered from a reduced functional viability of acinar cells in culture. As a result not only investigations on dispersed acinar cells and isolated acini were limited in their potential, but also prolonged studies on pancreatic exocrine and endocrine cells in an intact pancreatic tissue environment were unfeasible. To overcome these limitations, we aimed to establish a pancreas tissue slice culture platform to allow long-term studies on exocrine and endocrine cells in the intact pancreatic environment. Mouse pancreas tissue slice morphology was assessed to determine optimal long-term culture settings for intact pancreatic tissue. Utilizing optimized culture conditions, cell specificity and function of exocrine acinar cells and endocrine beta cells were characterized over a culture period of 7 days. We found pancreas tissue slices cultured under optimized conditions to have intact tissue specific morphology for the entire culture period. Amylase positive intact acini were present at all time points of culture and acinar cells displayed a typical strong cell polarity. Amylase release from pancreas tissue slices decreased during culture, but maintained the characteristic bell-shaped dose-response curve to increasing caerulein concentrations and a ca. 4-fold maximal over basal release. Additionally, endocrine beta cell viability and function was well preserved until the end of the observation period. Our results show that the tissue slice culture platform provides unprecedented maintenance of pancreatic tissue specific morphology and function over a culture period for at least 4 days and in part even up to 1 week. This analytical advancement now allows mid -to long-term studies on the cell biology of pancreatic disorder pathogenesis and therapy in an intact surrounding in situ.
Conflict of interest statement
Figures

References
-
- Bosco D, Soriano JV, Chanson M, Meda P (1994) Heterogeneity and contact-dependent regulation of amylase release by individual acinar cells. J Cell Physiol 160: 378–388. - PubMed
-
- Paraskevas S, Aikin R, Maysinger D, Lakey JR, Cavanagh TJ, et al. (1999) Activation and expression of ERK, JNK, and p38 MAP-kinases in isolated islets of Langerhans: implications for cultured islet survival. FEBS Lett 455: 203–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

