Recycling of the high valence States of heme proteins by cysteine residues of THIMET-oligopeptidase
- PMID: 24223886
- PMCID: PMC3815109
- DOI: 10.1371/journal.pone.0079102
Recycling of the high valence States of heme proteins by cysteine residues of THIMET-oligopeptidase
Abstract
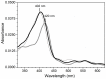
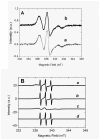
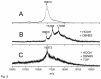
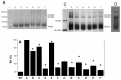
The peptidolytic enzyme THIMET-oligopeptidase (TOP) is able to act as a reducing agent in the peroxidase cycle of myoglobin (Mb) and horseradish peroxidase (HRP). The TOP-promoted recycling of the high valence states of the peroxidases to the respective resting form was accompanied by a significant decrease in the thiol content of the peptidolytic enzyme. EPR (electron paramagnetic resonance) analysis using DBNBS spin trapping revealed that TOP also prevented the formation of tryptophanyl radical in Mb challenged by H2O2. The oxidation of TOP thiol groups by peroxidases did not promote the inactivating oligomerization observed in the oxidation promoted by the enzyme aging. These findings are discussed towards a possible occurrence of these reactions in cells.
Conflict of interest statement
Figures


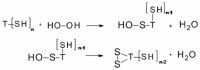


Similar articles
-
Effect of heme-apoprotein interactions on the activity of horseradish and wheat germ peroxidases.Biochem Biophys Res Commun. 1994 Oct 14;204(1):1-6. doi: 10.1006/bbrc.1994.2417. Biochem Biophys Res Commun. 1994. PMID: 7945347
-
Formation of long-lived radicals on proteins by radical transfer from heme enzymes--a common process?Arch Biochem Biophys. 1999 Feb 1;362(1):105-12. doi: 10.1006/abbi.1998.0988. Arch Biochem Biophys. 1999. PMID: 9917334
-
Role of tyrosine-103 in myoglobin peroxidase activity: kinetic and steady-state studies on the reaction of wild-type and variant recombinant human myoglobins with H(2)O(2).Biochemistry. 2002 Sep 24;41(38):11495-503. doi: 10.1021/bi025835w. Biochemistry. 2002. PMID: 12234193
-
Mechanisms of compound I formation in heme peroxidases.J Inorg Biochem. 2002 Jul 25;91(1):27-34. doi: 10.1016/s0162-0134(02)00390-2. J Inorg Biochem. 2002. PMID: 12121759 Review.
-
The Conundrum of Hydrogen Peroxide Signaling and the Emerging Role of Peroxiredoxins as Redox Relay Hubs.Antioxid Redox Signal. 2018 Mar 1;28(7):558-573. doi: 10.1089/ars.2017.7162. Epub 2017 Jul 17. Antioxid Redox Signal. 2018. PMID: 28587525 Review.
Cited by
-
Dimerization and thiol sensitivity of the salicylic acid binding thimet oligopeptidases TOP1 and TOP2 define their functions in redox-sensitive cellular pathways.Front Plant Sci. 2015 May 18;6:327. doi: 10.3389/fpls.2015.00327. eCollection 2015. Front Plant Sci. 2015. PMID: 26042129 Free PMC article.
-
Intermediate Tyrosyl Radical and Amyloid Structure in Peroxide-Activated Cytoglobin.PLoS One. 2015 Aug 27;10(8):e0136554. doi: 10.1371/journal.pone.0136554. eCollection 2015. PLoS One. 2015. PMID: 26312997 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

