N-cadherin relocalizes from the periphery to the center of the synapse after transient synaptic stimulation in hippocampal neurons
- PMID: 24223993
- PMCID: PMC3815108
- DOI: 10.1371/journal.pone.0079679
N-cadherin relocalizes from the periphery to the center of the synapse after transient synaptic stimulation in hippocampal neurons
Abstract
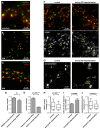
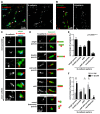
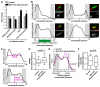
N-cadherin is a cell adhesion molecule which is enriched at synapses. Binding of N-cadherin molecules to each other across the synaptic cleft has been postulated to stabilize adhesion between the presynaptic bouton and the postsynaptic terminal. N-cadherin is also required for activity-induced changes at synapses, including hippocampal long term potentiation and activity-induced spine expansion and stabilization. We hypothesized that these activity-dependent changes might involve changes in N-cadherin localization within synapses. To determine whether synaptic activity changes the localization of N-cadherin, we used structured illumination microscopy, a super-resolution approach which overcomes the conventional resolution limits of light microscopy, to visualize the localization of N-cadherin within synapses of hippocampal neurons. We found that synaptic N-cadherin exhibits a spectrum of localization patterns, ranging from puncta at the periphery of the synapse adjacent to the active zone to an even distribution along the synaptic cleft. Furthermore, the N-cadherin localization pattern within synapses changes during KCl depolarization and after transient synaptic stimulation. During KCl depolarization, N-cadherin relocalizes away from the central region of the synaptic cleft to the periphery of the synapse. In contrast, after transient synaptic stimulation with KCl followed by a period of rest in normal media, fewer synapses have N-cadherin present as puncta at the periphery and more synapses have N-cadherin present more centrally and uniformly along the synapse compared to unstimulated cells. This indicates that transient synaptic stimulation modulates N-cadherin localization within the synapse. These results bring new information to the structural organization and activity-induced changes occurring at synapses, and suggest that N-cadherin relocalization may contribute to activity dependent changes at synapses.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

