Developmental switch in neurovascular coupling in the immature rodent barrel cortex
- PMID: 24224059
- PMCID: PMC3818260
- DOI: 10.1371/journal.pone.0080749
Developmental switch in neurovascular coupling in the immature rodent barrel cortex
Abstract
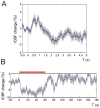
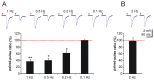
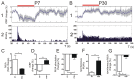
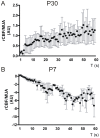
Neurovascular coupling (NVC) in the adult central nervous system (CNS) is a mechanism that provides regions of the brain with more oxygen and glucose upon increased levels of neural activation. Hemodynamic changes that go along with neural activation evoke a blood oxygen level-dependent (BOLD) signal in functional magnetic resonance imaging (fMRI) that can be used to study brain activity non-invasively. A correct correlation of the BOLD signal to neural activity is pivotal to understand this signal in neuronal development, health and disease. However, the function of NVC during development is largely unknown. The rodent whisker-to-barrel cortex is an experimentally well established model to study neurovascular interdependences. Using extracellular multi-electrode recordings and laser-Doppler-flowmetry (LDF) we show in the murine barrel cortex of postnatal day 7 (P7) and P30 mice in vivo that NVC undergoes a physiological shift during the first month of life. In the mature CNS it is well accepted that cortical sensory processing results in a rise in regional cerebral blood flow (rCBF). We show in P7 animals that rCBF decreases during prolonged multi-whisker stimulation and goes along with multi unit activity (MUA) fatigue. In contrast at P30, MUA remains stable during repetitive stimulation and is associated with an increase in rCBF. Further we characterize in both age groups the responses in NVC to single sensory stimuli. We suggest that the observed shift in NVC is an important process in cortical development that may be of high relevance for the correct interpretation of brain activity e.g. in fMRI studies of the immature central nervous system (CNS).
Conflict of interest statement
Figures



Similar articles
-
Impact of Altered Cholinergic Tones on the Neurovascular Coupling Response to Whisker Stimulation.J Neurosci. 2017 Feb 8;37(6):1518-1531. doi: 10.1523/JNEUROSCI.1784-16.2016. Epub 2017 Jan 9. J Neurosci. 2017. PMID: 28069927 Free PMC article.
-
Further nonlinearities in neurovascular coupling in rodent barrel cortex.Neuroimage. 2005 Jan 15;24(2):565-74. doi: 10.1016/j.neuroimage.2004.08.040. Neuroimage. 2005. PMID: 15627599
-
Neurovascular coupling is brain region-dependent.Neuroimage. 2012 Feb 1;59(3):1997-2006. doi: 10.1016/j.neuroimage.2011.09.050. Epub 2011 Sep 29. Neuroimage. 2012. PMID: 21982928
-
Neuronal networks and mediators of cortical neurovascular coupling responses in normal and altered brain states.Philos Trans R Soc Lond B Biol Sci. 2016 Oct 5;371(1705):20150350. doi: 10.1098/rstb.2015.0350. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27574304 Free PMC article. Review.
-
Integrated Feedforward and Feedback Mechanisms in Neurovascular Coupling.Anesth Analg. 2024 Dec 1;139(6):1283-1293. doi: 10.1213/ANE.0000000000006891. Epub 2024 Feb 12. Anesth Analg. 2024. PMID: 38345932 Review.
Cited by
-
Neurovascular coupling and energy metabolism in the developing brain.Prog Brain Res. 2016;225:213-42. doi: 10.1016/bs.pbr.2016.02.002. Epub 2016 Mar 22. Prog Brain Res. 2016. PMID: 27130418 Free PMC article.
-
Interpreting BOLD: towards a dialogue between cognitive and cellular neuroscience.Philos Trans R Soc Lond B Biol Sci. 2016 Oct 5;371(1705):20150348. doi: 10.1098/rstb.2015.0348. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27574302 Free PMC article.
-
Binaural blood flow control by astrocytes: listening to synapses and the vasculature.J Physiol. 2017 Mar 15;595(6):1885-1902. doi: 10.1113/JP270979. Epub 2016 Oct 14. J Physiol. 2017. PMID: 27619153 Free PMC article. Review.
-
Postnatal development of cerebrovascular structure and the neurogliovascular unit.Wiley Interdiscip Rev Dev Biol. 2020 Mar;9(2):e363. doi: 10.1002/wdev.363. Epub 2019 Oct 1. Wiley Interdiscip Rev Dev Biol. 2020. PMID: 31576670 Free PMC article. Review.
-
Developmental Changes in Sensory-Evoked Optical Intrinsic Signals in the Rat Barrel Cortex.Front Cell Neurosci. 2017 Dec 12;11:392. doi: 10.3389/fncel.2017.00392. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29311827 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

