Mechanisms of load dependency of myocardial ischemia reperfusion injury
- PMID: 24224132
- PMCID: PMC3819580
Mechanisms of load dependency of myocardial ischemia reperfusion injury
Abstract
Coronary artery disease and associated ischemic heart disease are prevalent disorders worldwide. Further, systemic hypertension is common and markedly increases the risk for heart disease. A common denominator of systemic hypertension of various etiologies is increased myocardial load/mechanical stress. Thus, it is likely that high pressure/mechanical stress attenuates the contribution of cardioprotective but accentuates the contribution of cardiotoxic pathways thereby exacerbating the outcome of an ischemia reperfusion insult to the heart. Critical events which contribute to cardiomyocyte injury in the ischemic-reperfused heart include cellular calcium overload and generation of reactive oxygen/nitrogen species which, in turn, promote the opening of the mitochondrial permeability transition pore, an important event in cell death. Increasing evidence also indicates that the myocardium is capable of mounting a robust inflammatory response which contributes importantly to tissue injury. On the other hand, cardioprotective maneuvers of ischemic preconditioning and postconditioning have led to identification of complex web of signaling pathways (e.g., reperfusion injury salvage kinase) which ultimately converge on the mitochondria to exert cytoprotection. The present review is intended to briefly describe mechanisms of cardiac ischemia reperfusion injury followed by a discussion of our work focused on how pressure/mechanical stress modulates endogenous cardiotoxic and cardioprotective mechanisms to ultimately exacerbate ischemia reperfusion injury.
Keywords: Heart; calcium overload; inflammation; ischemia-reperfusion; oxidative/nitrosative stress; pressure; signaling mechanisms; stem cells.
Figures
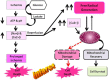
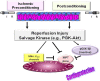
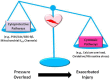
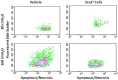
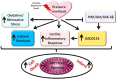
References
-
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. - PubMed
-
- Deaton C, Froelicher ES, Wu LH, Ho C, Shishani K, Jaarsma T. The global burden of cardiovascular disease. J Cardiovasc Nurs. 2011;26(Suppl 4):S5–14. - PubMed
-
- Arima H, Barzi F, Chalmers J. Mortality patterns in hypertension. J Hypertens. 2011;29(Suppl 1):S3–S7. - PubMed
-
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Michol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive Summary: Heart Disease and Stroke Statistics—2013 Update: A Report From the American Heart Association. Circulation. 2013;127:143–152. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
