DNA ligases as therapeutic targets
Abstract
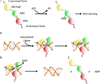
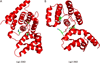
During DNA replication, DNA joining events link Okazaki fragments on the lagging strand. In addition, they are required to repair DNA single- and double-strand breaks and to complete repair events initiated by the excision of mismatched and damaged bases. In human cells, there are three genes encoding DNA ligases. These enzymes are ATP-dependent and contain a conserved catalytic region. Biophysical studies have shown that the catalytic region contains three domains that, in the absence of DNA, are in an extended conformation. When the catalytic region engages a DNA nick, it adopts a compact, ring structure around the DNA nick with each of the three domains contacting the DNA. Protein-protein interactions involving the regions flanking the conserved catalytic regions of human DNA ligases play a major role in directing these enzymes to participate in specific DNA transactions. Among the human LIG genes, the LIG3 gene is unique in that it encodes multiple DNA ligase polypeptides with different N- and C-termini. One of these polypeptides is targeted to mitochondria where it plays an essential role in the maintenance of the mitochondrial genome. In the nucleus, DNA ligases I, III and IV have distinct but overlapping functions in DNA replication and repair. Small molecule inhibitors of human DNA ligases have been identified using structure-based approaches. As expected, these inhibitors are cytotoxic and also potentiate the cytotoxicity of DNA damaging agents. The results of preclinical studies with human cancer cell lines and mouse models of human cancer suggest that DNA ligase inhibitors may have utility as anti-cancer agents.
Keywords: Cancer; DNA ligase; DNA repair; DNA replication; genome instability; mitochondria.
Conflict of interest statement
Figures


References
-
- Friedberg EC, Walker GC, Siede W, et al. DNA Repair and Mutagenesis. 2nd ed. ASM Press; 2006.
-
- Lord CJ, Ashworth A. Targeted therapy for cancer using PARP inhibitors. Curr Opin Pharmacol. 2008;8:363–369. - PubMed
-
- Pascal JM, O’Brien PJ, Tomkinson AE, et al. Human DNA ligase I completely encircles and partially unwinds nicked DNA. Nature. 2004;432:473–478. - PubMed
-
- Tomkinson AE, Lasko DD, Daly G, et al. Mammalian DNA ligases. Catalytic domain and size of DNA ligase I. J Biol Chem. 1990;265:12611–12617. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
