Medication management for people with dementia in primary care: description of implementation in the DelpHi study
- PMID: 24225205
- PMCID: PMC3840668
- DOI: 10.1186/1471-2318-13-121
Medication management for people with dementia in primary care: description of implementation in the DelpHi study
Abstract
Background: As the population ages, the relative and absolute number of age-associated diseases such as dementia will increase. Evaluation of the suitability and intake of medication and pharmacological treatment is an important aspect of care for people with dementia, especially if they live at home. Regular medication reviews and systematic cooperation between physicians and pharmacists are not common in routine care. Medication management (MM), based on such a comprehensive home medication review could help to reduce drug-related problems and costs. The present article presents a medication management specifically for the application in the ambulatory setting and describes its implementation as part of a larger trial.
Methods/design: A home medication review (HMR) and MM is implemented as part of the DelpHi study, a population based prospective, cluster-randomized controlled intervention study to test the efficacy and efficiency of the implementation of a collaborative care model in primary care.
Participants: people with dementia (PWD) and their caregivers are recruited by the patient's general practitioner. Inclusion criteria are a positive screening result for dementia, living at home and regular intake of drugs. PWD are asked to specify their regular pharmacy which is asked to participate in the study, too.
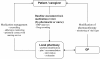
Intervention: a comprehensive HMR is conducted as computer-assisted personal interview by specifically qualified Dementia Care Manager (DCM) at the people's home. It includes detailed information about drugs taken, their storage, administration, adherence and adverse events. The MM is conducted in cooperation between DCM, pharmacist and general practitioner and consists of a pharmaceutical evaluation, pharmaceutical recommendations and their application. Pharmacists are trained and provided with regularly updated information. The MM is designed to give information and recommendations concerning antidementia drugs, occurrence of drug related problems, intake of anticholinergic drugs, potentially clinically relevant drug-drug-interactions, adverse drug events and medication adherence.
Discussion: The DelpHi-approach for medication management employs comprehensive instruments and procedures in the primary care setting under routine care conditions, and this approach should be useful in improving pharmacotherapy as part of the comprehensive treatment and care for people with dementia.
Trial registration: The trial is registered at ClinicalTrials.gov, number NCT01401582.
Figures
References
-
- Siewert U, Fendrich K, Doblhammer-Reiter G, Scholz RD, Schuff-Werner P, Hoffmann W. Health care consequences of demographic changes in Mecklenburg-West Pomerania: projected case numbers for age-related diseases up to the year 2020, based on the study of health in Pomerania (SHIP) Dtsch Arztebl Int. 2010;107:328–334. - PMC - PubMed
-
- Thyrian JR, Dreier A, Fendrich K, Lueke S, Hoffmann W. Demenzerkrankungen–Wirksame Konzepte gesucht. Deutsches Ärzteblatt. 2011;108:A1954–A1956.
-
- National Institute for Health and Clinical Excellence. NICE clinical guideline. 42. NICE; 2006. (Dementia supporting people with dementia and their carers in health and social care). http://publications.nice.org.uk/dementia-cg42.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials