Human immunodeficiency virus type 1 viral protein R (Vpr) induces CCL5 expression in astrocytes via PI3K and MAPK signaling pathways
- PMID: 24225433
- PMCID: PMC3831867
- DOI: 10.1186/1742-2094-10-136
Human immunodeficiency virus type 1 viral protein R (Vpr) induces CCL5 expression in astrocytes via PI3K and MAPK signaling pathways
Abstract
Background: Neurocognitive impairments remain prevalent in HIV-1 infected individuals despite current antiretroviral therapies. It is increasingly becoming evident that astrocytes play a critical role in HIV-1 neuropathogenesis through the production of proinflammatory cytokines/chemokines. HIV-1 viral protein R (Vpr) plays an important role in neuronal dysfunction; however, its role in neuroinflammation is not well characterized. The major objective of this study was to determine the effect of Vpr in induction of proinflammatory chemokine CCL5 in astrocytes and to define the underlying mechanism(s).
Methods: SVGA astrocytes were either mock transfected or were transfected with a plasmid encoding HIV-1 Vpr, and the cells were harvested at different time intervals. The mRNA level of CCL5 expression was quantified using real-time RT-PCR, and cell culture supernatants were assayed for CCL5 protein concentration. Immunocytochemistry was performed on HIV-1 Vpr transfected astrocytes to check CCL5 expression. Various signaling mechanisms such as p38 MAPK, PI3K/Akt, NF-κB and AP-1 were explored using specific chemical inhibitors and siRNAs.
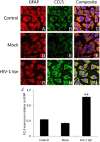
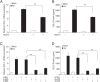
Results: HIV-1 Vpr transfected astrocytes exhibited time-dependent induction of CCL5 as compared to mock-transfected astrocytes at both the mRNA and protein level. Immunostained images of astrocytes transfected with HIV-1 Vpr also showed much higher accumulation of CCL5 in comparison to untransfected and mock-transfected astrocytes. Pre-treatment with NF-κB (SC514) and PI3K/Akt (LY294002) inhibitor partially abrogated CCL5 mRNA and protein expression levels as opposed to untreated controls after HIV-1 Vpr transfection. Specific siRNAs against p50 and p65 subunits of NF-κB, p38δ MAPK, Akt-2 and Akt-3, and AP-1 transcription factor substantially inhibited the production of CCL5 in HIV-1 Vpr transfected astrocytes.
Conclusion: These results demonstrate the ability of HIV-1 Vpr to induce CCL5 in astrocytes in a time-dependent manner. Furthermore, this effect was observed to be mediated by transcription factors NF-κB and AP-1 and involved the p38-MAPK and PI3K/Akt pathway.
Figures
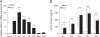



Similar articles
-
Viral protein R (Vpr)-induced neuroinflammation and its potential contribution to neuronal dysfunction: a scoping review.BMC Infect Dis. 2023 Aug 6;23(1):512. doi: 10.1186/s12879-023-08495-3. BMC Infect Dis. 2023. PMID: 37545000 Free PMC article.
-
HIV-1 Tat-mediated induction of CCL5 in astrocytes involves NF-κB, AP-1, C/EBPα and C/EBPγ transcription factors and JAK, PI3K/Akt and p38 MAPK signaling pathways.PLoS One. 2013 Nov 11;8(11):e78855. doi: 10.1371/journal.pone.0078855. eCollection 2013. PLoS One. 2013. PMID: 24244375 Free PMC article.
-
HIV-1 Nef induces CCL5 production in astrocytes through p38-MAPK and PI3K/Akt pathway and utilizes NF-kB, CEBP and AP-1 transcription factors.Sci Rep. 2014 Mar 24;4:4450. doi: 10.1038/srep04450. Sci Rep. 2014. PMID: 24658403 Free PMC article.
-
Molecular mechanisms involved in HIV-1 Tat-mediated induction of IL-6 and IL-8 in astrocytes.J Neuroinflammation. 2014 Dec 24;11:214. doi: 10.1186/s12974-014-0214-3. J Neuroinflammation. 2014. PMID: 25539898 Free PMC article.
-
Multiple Protein Kinases via Activation of Transcription Factors NF-κB, AP-1 and C/EBP-δ Regulate the IL-6/IL-8 Production by HIV-1 Vpr in Astrocytes.PLoS One. 2015 Aug 13;10(8):e0135633. doi: 10.1371/journal.pone.0135633. eCollection 2015. PLoS One. 2015. PMID: 26270987 Free PMC article.
Cited by
-
Viral protein R (Vpr)-induced neuroinflammation and its potential contribution to neuronal dysfunction: a scoping review.BMC Infect Dis. 2023 Aug 6;23(1):512. doi: 10.1186/s12879-023-08495-3. BMC Infect Dis. 2023. PMID: 37545000 Free PMC article.
-
Friends Turn Foe-Astrocytes Contribute to Neuronal Damage in NeuroAIDS.J Mol Neurosci. 2019 Oct;69(2):286-297. doi: 10.1007/s12031-019-01357-1. Epub 2019 Jun 25. J Mol Neurosci. 2019. PMID: 31236774 Review.
-
Macrophage migration inhibitory factor facilitates production of CCL5 in astrocytes following rat spinal cord injury.J Neuroinflammation. 2018 Sep 4;15(1):253. doi: 10.1186/s12974-018-1297-z. J Neuroinflammation. 2018. PMID: 30180853 Free PMC article.
-
Defining the roles for Vpr in HIV-1-associated neuropathogenesis.J Neurovirol. 2016 Aug;22(4):403-15. doi: 10.1007/s13365-016-0436-5. Epub 2016 Apr 7. J Neurovirol. 2016. PMID: 27056720 Free PMC article. Review.
-
Intracellular Redox-Modulated Pathways as Targets for Effective Approaches in the Treatment of Viral Infection.Int J Mol Sci. 2021 Mar 30;22(7):3603. doi: 10.3390/ijms22073603. Int J Mol Sci. 2021. PMID: 33808471 Free PMC article. Review.
References
-
- Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH. et al.HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. - DOI - PMC - PubMed
-
- Adle-Biassette H, Chretien F, Wingertsmann L, Hery C, Ereau T, Scaravilli F, Tardieu M, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol. 1999;25(2):123–133. doi: 10.1046/j.1365-2990.1999.00167.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

