Shape selectivity and remapping in dorsal stream visual area LIP
- PMID: 24225538
- PMCID: PMC3921402
- DOI: 10.1152/jn.00841.2011
Shape selectivity and remapping in dorsal stream visual area LIP
Abstract
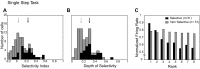
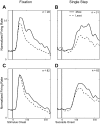
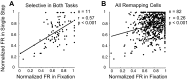
We explore the visual world by making rapid eye movements (saccades) to focus on objects and locations of interest. Despite abrupt retinal image shifts, we see the world as stable. Remapping contributes to visual stability by updating the internal image with every saccade. Neurons in macaque lateral intraparietal cortex (LIP) and other brain areas update information about salient locations around the time of a saccade. The depth of information transfer remains to be thoroughly investigated. Area LIP, as part of the dorsal visual stream, is regarded as a spatially selective area, yet there is evidence that LIP neurons also encode object features. We sought to determine whether LIP remaps shape information. This knowledge is important for understanding what information is retained from each glance. We identified 82 remapping neurons. First, we presented shapes within the receptive field and tested for shape selectivity in a fixation task. Among the remapping neurons, 28 neurons (34%) were selective for shape. Second, we presented the same shapes in the future location of the receptive field around the time of the saccade and tested for shape selectivity during remapping. Thirty-one (38%) neurons were selective for shape. Of 11 neurons that were shape selective in both tasks, 5 showed significant correlation between shape selectivity in the two tasks. Across the population, there was a weak but significant correlation between responses to shape in the two tasks. Our results provide neurophysiological evidence that remapped responses in area LIP can encode shape information as well as spatial information.
Keywords: monkey; parietal; spatial updating.
Figures




References
-
- Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20: 303–330, 1997 - PubMed
-
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. I. Temporal properties; comparison with area 7a. J Neurophysiol 66: 1095–1108, 1991a - PubMed
-
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. II. Spatial properties. J Neurophysiol 66: 1109–1124, 1991b - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

