A novel approach for separating bacteriophages from other bacteriophages using affinity chromatography and phage display
- PMID: 24225840
- PMCID: PMC3827602
- DOI: 10.1038/srep03220
A novel approach for separating bacteriophages from other bacteriophages using affinity chromatography and phage display
Abstract
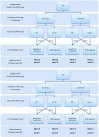
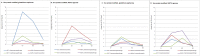
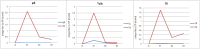
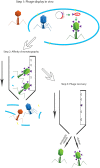
Practical applications of bacteriophages in medicine and biotechnology induce a great need for technologies of phage purification. None of the popular methods offer solutions for separation of a phage from another similar phage. We used affinity chromatography combined with competitive phage display (i) to purify T4 bacteriophage from bacterial debris and (ii) to separate T4 from other contaminating bacteriophages. In 'competitive phage display' bacterial cells produced both wild types of the proteins (expression from the phage genome) and the protein fusions with affinity tags (expression from the expression vectors). Fusion proteins were competitively incorporated into the phage capsid. It allowed effective separation of T4 from a contaminating phage on standard affinity resins.
Conflict of interest statement
K.D., P.M., A.P., B.O., A.G. are inventors of a patent (patent application no P 392774) owned by the Institute of Immunology and Experimental Therapy related to phage purification. I.C., D.L., K.H., M.H. declare no potential conflict of interest.
Figures

References
-
- McLaughlin M. R. & King R. A. Characterization of Salmonella bacteriophages isolated from swine lagoon effluent. Curr. Microbiol. 56, 208–213 (2008). - PubMed
-
- Boratyński J. et al. Preparation of endotoxin-free bacteriophages. Cell Mol Biol Lett. 9, 253–9 (2004). - PubMed
-
- Brorson K., Shen H., Lute S., Pérez J. S. & Frey D. D. Characterization and purification of bacteriophages using chromatofocusing. J Chromatogr A. 1207, 110–21 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

